Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
Regulatory Collaboration and System Strengthening – Workshop Synopsis
This CIRS multi-stakeholder workshop examined success factors for strengthening regulatory systems to support the implementation of collaborative models.
Workshop Report – Regulatory and HTA collaborative models
In this workshop, CIRS brought together senior representatives from regulators, HTA agencies, pharmaceutical companies, payers, academics and patient organisations to discuss the impact of regulatory and HTA collaborative models [...]
Ensuring efficiency and effectiveness of Joint Clinical Assessment (JCA) – Wang 2025
Background: This study explored the readiness and strategic considerations of companies and key stakeholders for the implementation of the Joint Clinical Assessment (JCA) under the European Health Technology Assessment [...]
CIRS RD Briefing 97 – Access Consortium and Project Orbis Approvals Across Eight Regulators
This R&D Briefing builds upon the Centre for Innovation in Regulatory Science (CIRS)'s long-standing efforts to examine trends and practices in regulatory approvals. For over 20 years, CIRS has [...]
Economic impact of reliance on an African regulator – Danks 2025
Background and Objectives The inherited backlog of 16,000 medicines applications of the South African Health Products Regulatory Authority (SAHPRA) was cleared through facilitated review pathways that included reliance on [...]
Review of 2024 CIRS activities
We're pleased to share a high-level summary of what CIRS got up to last year, including key research outputs and meetings. Our full 2024 Annual Report will be published [...]
Suggested Improvements to the EAC-MRH Joint Review Process – Ngum 2025
Background In 2012, the East African Community Medicines Regulatory Harmonization (EAC-MRH) initiative was established to improve access to safe, effective, and high-quality medical products to patients in the East [...]
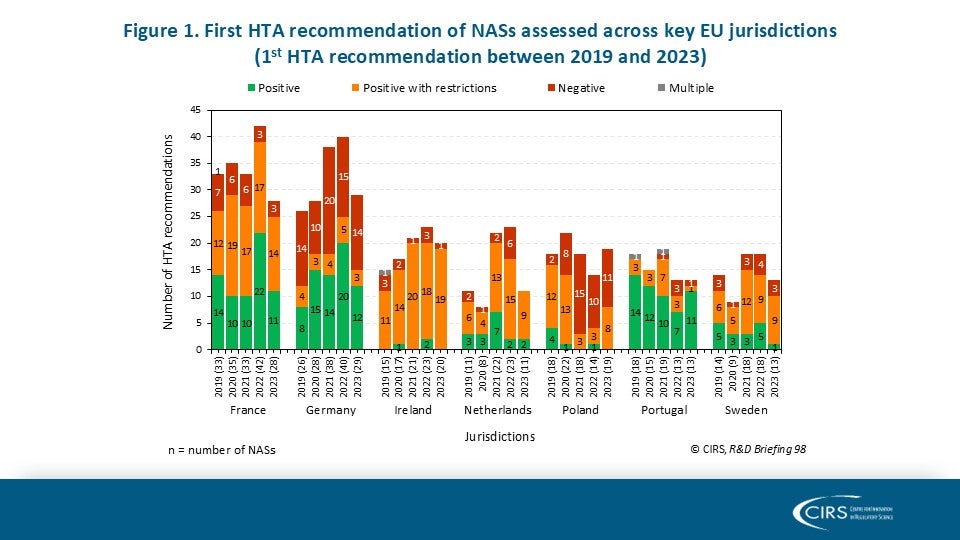
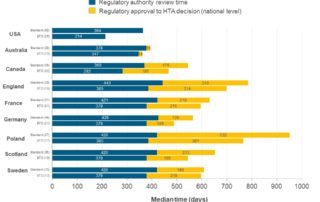
CIRS RD Briefing 98 – European HTA trends: HTA outcomes and timelines across seven markets 2019-2023
This R&D Briefing presents data from HTADock, an ongoing CIRS metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies. It [...]
Comparison of good review practices in ECOWAS – Owusu-Asante 2025
Introduction: When implemented by national and regional regulatory agencies good review practices (GRevPs) support the timely high-quality review of medicines for enhanced patients’ availability to safe, quality and efficacious [...]
Evaluation of Zambian Medicines Regulatory Authority review process – Chisha 2024
Purpose: This study aimed to assess the current regulatory review process of the Zambia Medicines Regulatory Authority (ZAMRA) by identifying the key milestones and target timelines achieved for products [...]
Workshop Synopsis – Regulatory and HTA collaborative models
In this workshop, CIRS brought together senior representatives from regulators, HTA agencies, pharmaceutical companies, payers, academics and patient organisations to discuss the impact of regulatory and HTA collaborative models [...]
Frequency and Variation of Clock-Stop During EMA Assessment for Oncology Products – Implication on JCA Timelines
Objectives: The implementation act adopted for the HTA Regulation (HTAR) defined the timelines of scoping, submission and assessment and output of Joint Clinical Assessment (JCA), which will be in [...]
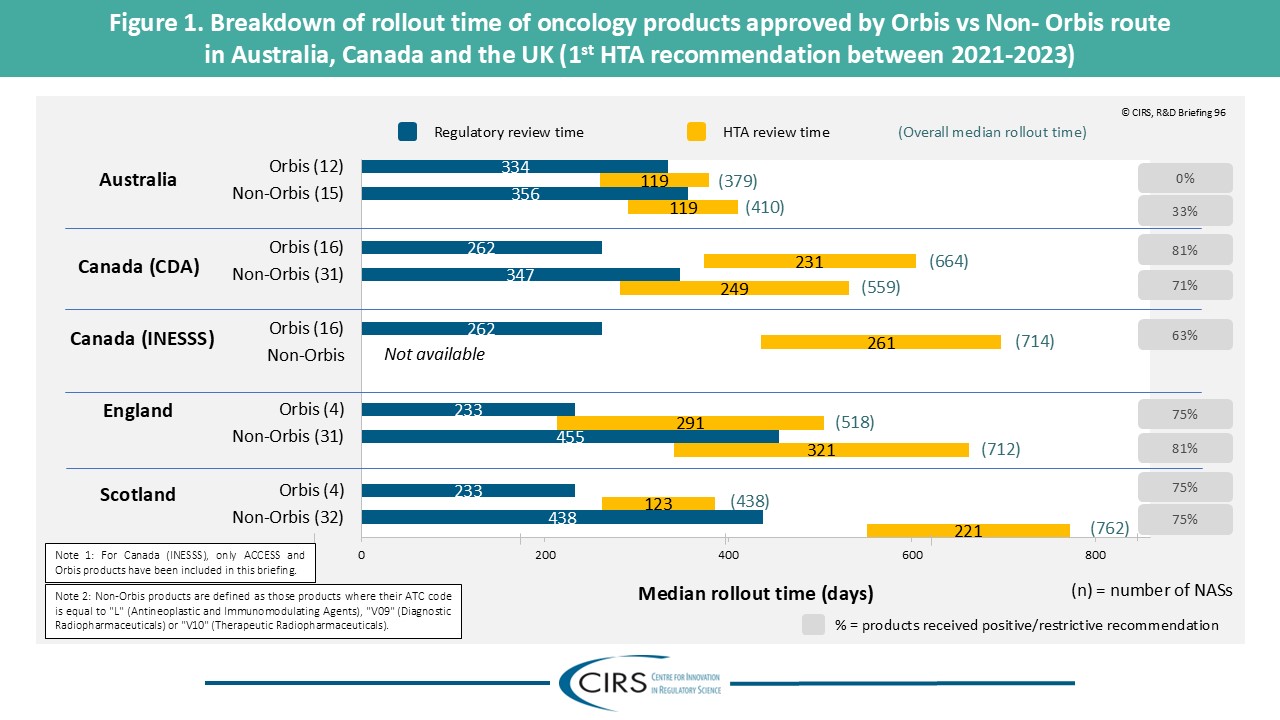
CIRS RD Briefing 96 – Review of HTA outcomes and timelines in Australia, Canada and the UK 2019-2023
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies. It focuses [...]
Ngum 2024 – Evaluation of review models and timelines in the EAC-MRH
Introduction: Medicines regulatory harmonisation has been embraced by many national regulatory authorities (NRAs) to improve public health through faster availability of safe, high-quality, and effective medical products to patients [...]
Ngum 2024 – Evaluation of good review practices in the EAC-MRH
Introduction: The East African Community Medicines Regulatory Harmonisation (EAC-MRH) programme was established to address challenges faced by national regulatory authorities (NRAs) of the region. Work sharing through joint assessments [...]
Workshop Report – Vaccine regulatory and funding approaches
Over the last four years there has been much greater attention paid by industry, regulators, health technology assessment (HTA) bodies and the general public to vaccines for a range [...]
Monitoring implementation and adherence to ICH guidelines
Background: This study was built on the previous 2019 and 2021 assessments where the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) selected CIRS [...]
Workshop Report – Ensuring efficient and effective implementation of joint clinical assessment (JCA)
The Regulation (EU) 2021/2282 on health technology assessment (HTAR) reflects a significant step towards harmonising the clinical assessment in HTA decision making across EU Member States. It aims to [...]
2024 Workshop Synopsis – Evolving regulatory and funding approaches for vaccines
In this workshop, CIRS brought together senior representatives from the international pharmaceutical industry, regulatory agencies, National Immunisation Technical Advisory Groups (NITAGs), HTA agencies, payers and academia to identify challenges [...]
Role of Regional Initiatives in the Operationalisation of the African Medicines Agency: Contribution of the EAC-MRH Initiative
“This book teaches us important lessons that we will need to consider in our collaboration work with the African Medicines Agency. I am confident that this research will be [...]
2024 Workshop Synopsis – Ensuring efficient and effective implementation of joint clinical assessment (JCA)
In this workshop, CIRS brought together senior representatives from HTA agencies, pharmaceutical companies, payers and patient organisations to discuss their readiness for the EU HTA Regulation (HTAR) being applied [...]
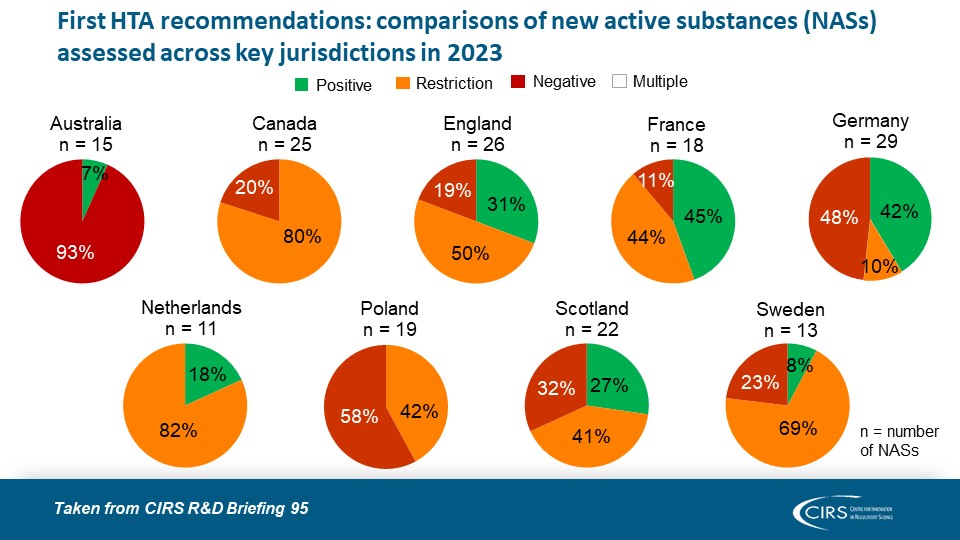
CIRS RD Briefing 95 – Review of HTA outcomes and timelines in Australia, Canada, Europe and the UK 2019-2023
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies, each with [...]
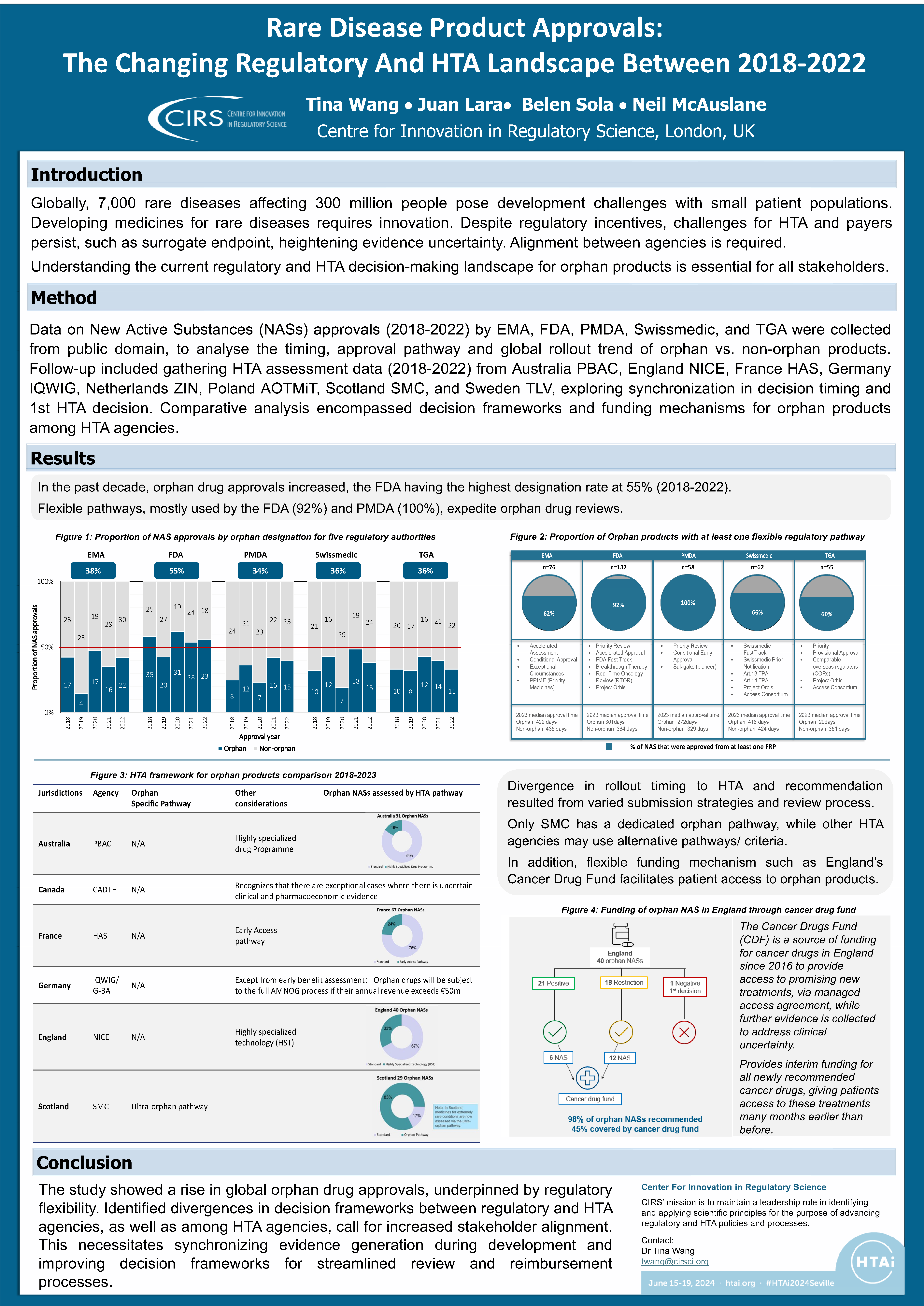
Rare Disease Product Approvals: The Changing Regulatory And HTA Landscape Between 2018-2022
Background Globally, 7,000 rare diseases affecting 300 million people pose development challenges with small patient populations. Developing medicines for rare diseases requires innovation. Despite regulatory incentives, challenges for HTA [...]
2024 Workshop report – Reliance and regional review models
Faced with increasingly complex technologies and novel evidence generation techniques, regulatory agencies are being challenged to work in new ways. There is pressure on them to be agile and [...]
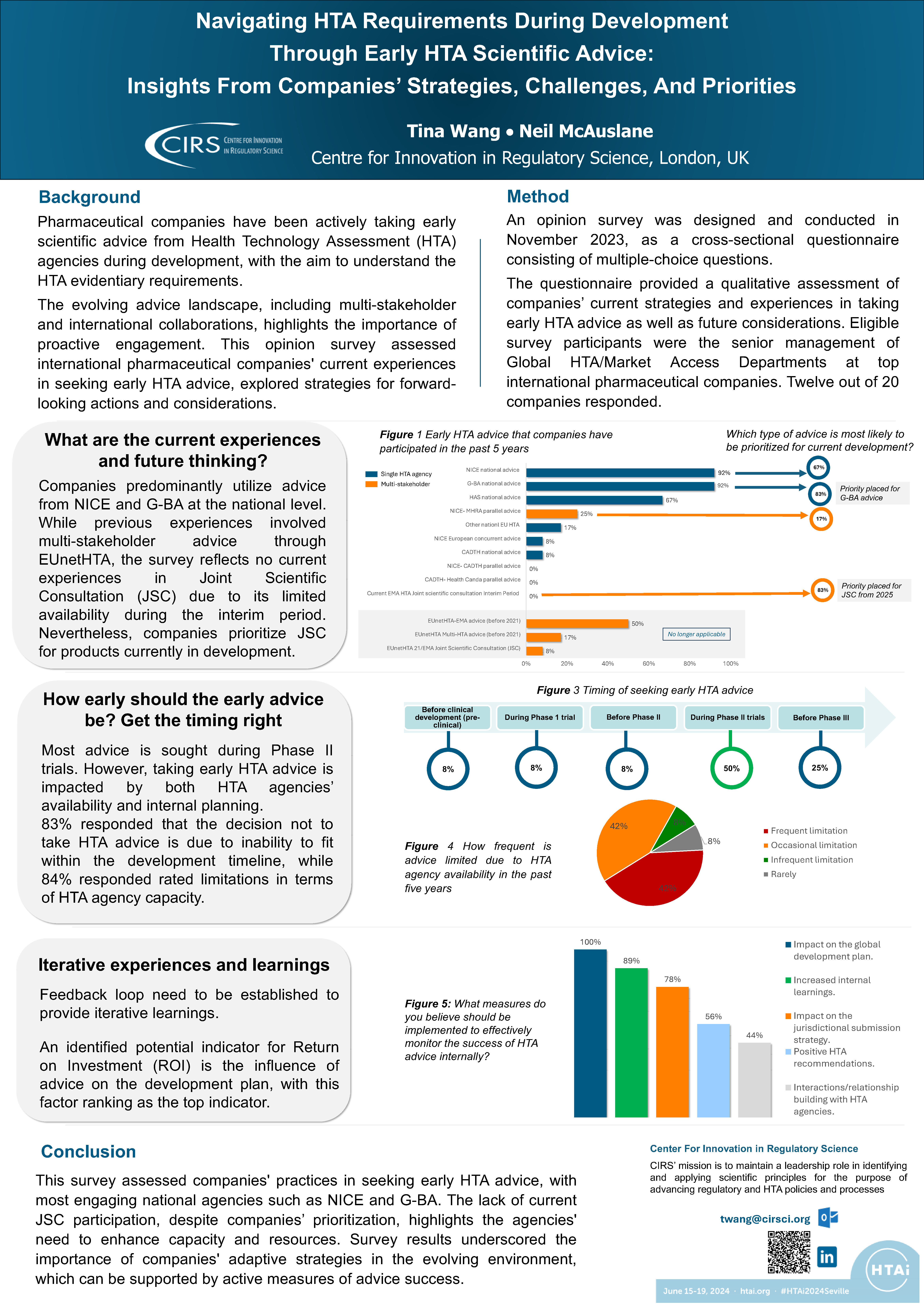
Navigating HTA Requirements During Development Through Early HTA Scientific Advice
Background Pharmaceutical companies have been actively taking early scientific advice from health technology assessment (HTA) agencies during development, with the aim to understand the HTA evidentiary requirements. The evolving [...]
Comparison of Three Regional Medicines Regulatory Harmonisation Initiatives in Africa
Background The African Medicines Regulatory Harmonisation (AMRH) Initiative was formed in 2009 and subsequently, three regional initiatives (East African Community Medicines Regulatory Harmonisation [MRH], Southern African Development Community [SADC]/ZaZiBoNa [...]
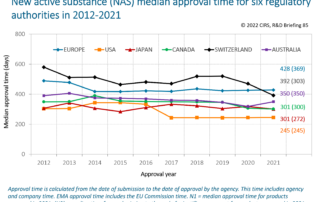
CIRS RD Briefing 93 – New drug approvals by six major authorities 2014-2023
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the [...]
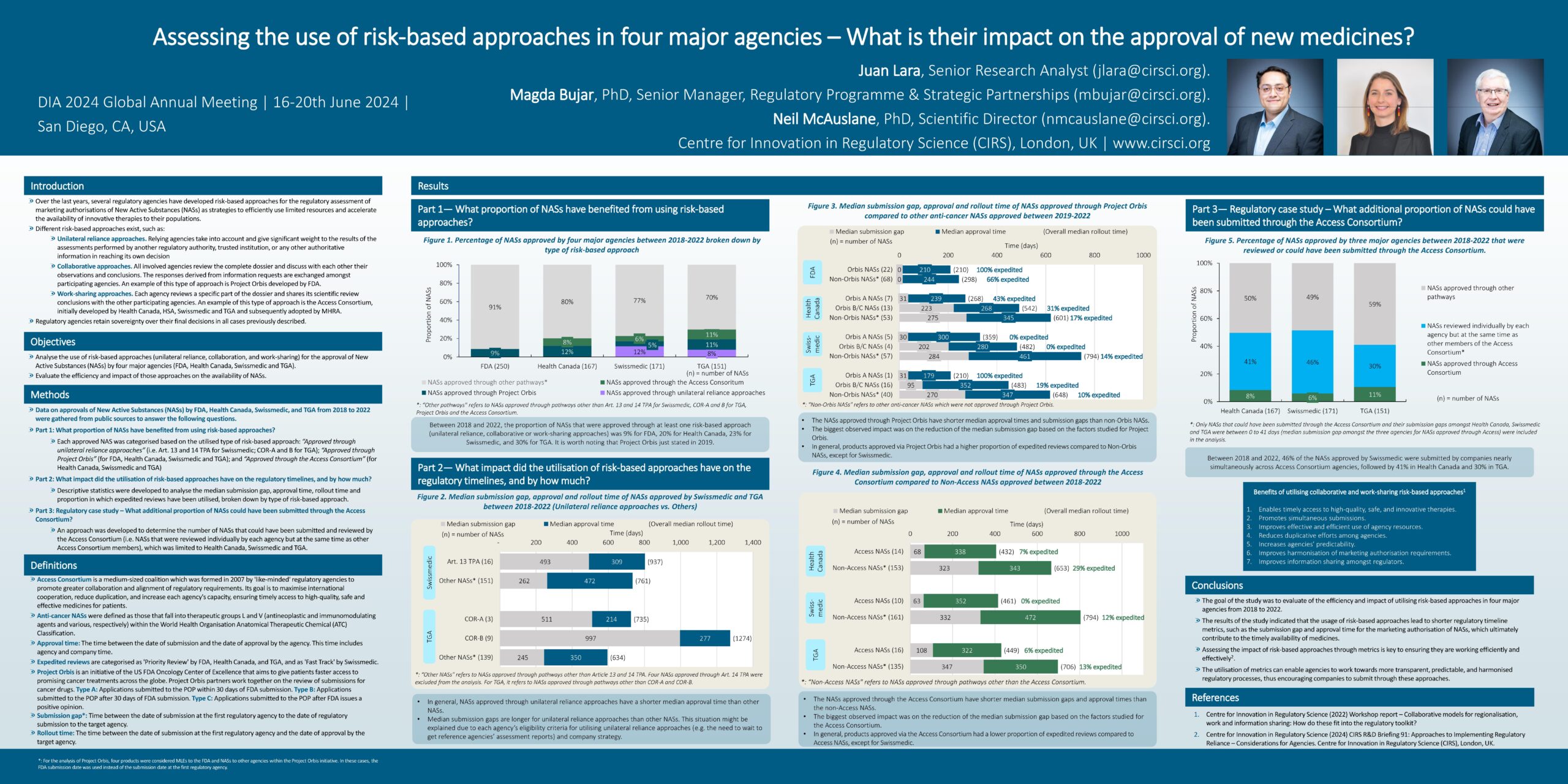
Assessing the use of risk-based approaches in four major agencies
Introduction Over the last years, several regulatory agencies have developed risk-based approaches for the regulatory assessment of marketing authorisations of New Active Substances (NASs) as strategies to efficiently use [...]
CIRS 2023 Annual Report
We're delighted to present our latest Annual Report, which provides a summary of the projects, publications, multi-stakeholder workshops and Technical Fora from Regulatory and HTA workstreams undertaken in 2023. [...]
CIRS RD Briefing 94 – Value of Reference Agency Reports in Enabling Reliance
Access to information, including the assessment documents of reference national regulatory agencies (NRA), is a key enabler of regulatory risk-based decision making. It promotes an understanding of what was [...]
2024 Workshop Synopsis – What is needed for risk-based approaches to work effectively and efficiently?
In this workshop, CIRS brought together senior representatives from regulatory agencies, pharmaceutical companies and academia from 18 countries across the Americas, Africa, Asia and Europe, to examine risk-based approaches [...]
CIRS RD Briefing 92 – Appraising the usability of public assessment reports for reliance
Regulatory reliance facilitates regulatory approvals, allows the use of resources more efficiently, and ultimately serves patients by accelerating access to quality-assured, safe, and effective medicines. The World Health Organisation [...]
2023 Workshop report – Review and reimbursement frameworks for rare disease products
There are an estimated 300 million people across the world affected by around 7000 known rare diseases. Challenges in bringing treatments to market for these conditions include small patient [...]
2023 Workshop report – Uncertainty in the development of new medicines
This CIRS workshop brought together companies and agencies (HTA and Regulatory) to discuss the sources of uncertainty that are being built in, by the way medicines development has evolved [...]
2023 Workshop Synopsis – Regulatory and reimbursement frameworks for rare disease products
This multi-stakeholder workshop consisted of a series of presentation sessions and three parallel breakout discussions. Presentations explored trends in regulatory and HTA approvals of orphan products [...]
CIRS RD Briefing 91 – Approaches to Implementing Regulatory Reliance: Considerations for Agencies
This CIRS briefing delves into the increasingly pivotal role of regulatory reliance in the global pharmaceutical landscape. Reliance is defined by World Health Organization (WHO) as the act whereby [...]
2023 Workshop report – New ways of working for medicines development
CIRS brought agencies and companies together in a workshop to discuss new ways of working and how the regulatory and HTA landscape in mature and maturing countries should evolve [...]
HTA Timelines and Outcomes for MHRA-Approved NASs via Reliance/Work-sharing Routes
During ISPOR Europe 2023 in Copenhagen, Belen Sola presented a poster entitled ‘Study of HTA Timelines and Outcomes for MHRA-Approved NASs in the Post-Brexit UK via Reliance/Work-sharing Routes'. Background: [...]
Evaluation of the Swissmedic regulatory framework for new active substances
Background: Swissmedic is a major regulatory agency that has been benchmarking its timelines for 20 years. To better understand the Swissmedic review times and to examine whether measures introduced to [...]
CIRS RD Briefing 90 – Challenges and opportunities for orphan medicines availability in Mexico
The document ‘Estrategia sobre Certidumbre Regulatoria para el Sector Farmacéutico’ (Strategy of Regulatory Certitude for the Pharmaceutical Sector), published last January by COFEPRIS describes important working projects the agency [...]
Impact of reliance on the regulatory performance of the South African Health Products Regulatory Authority
Introduction: The World Health Organization (WHO) advocates the use of reliance practices to enable national regulatory authorities (NRAs) to improve patients’ access to medicines. This study considered whether reliance review [...]
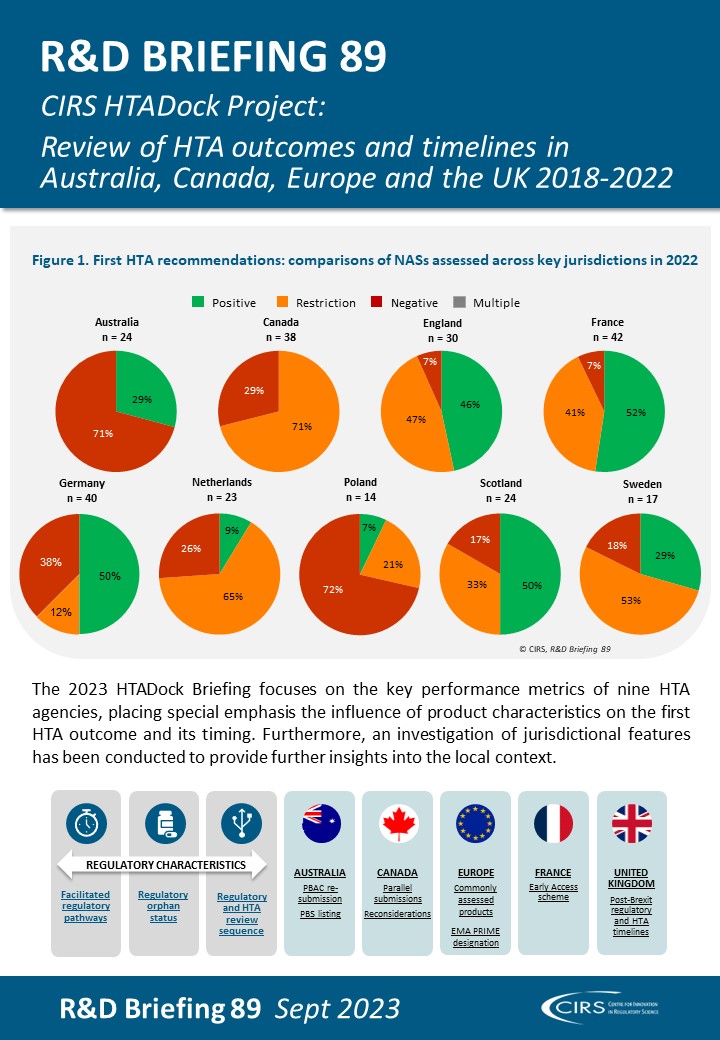
CIRS RD Briefing 89 – Review of HTA outcomes and timelines in Australia, Canada, Europe and the UK 2018-2022
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies, each with [...]
Measuring time to market for new medicines in 7 Asian countries between 2016-21, following review by US FDA or EMA
During the DIA Global Annual Meeting 2023, Adem Kermad, Magda Bujar and Neil McAuslane developed and presented a poster in which they shared the results, recommendations and conclusions about [...]
Evaluation of the Effectiveness and Efficiency of Ten Years’ Experience with the East Africa Community Joint Assessment
During the DIA Global Annual Meeting 2023, Nancy Yang-Ngum developed and presented a poster in which she shared the results, recommendations and conclusions about the topic "Evaluation of the [...]
Evaluation of the Regulatory Review Process of the FDA Ghana: Challenges and Opportunities for Improvement
During the DIA Global Annual Meeting 2023, Mercy Owusu-Asante developed and presented a poster in which she shared the results, recommendations and conclusions about the topic "Evaluation of the [...]
Evaluation of the impact of reliance on the regulatory performance in the South African Health Products Regulatory Authority
During the DIA Global Annual Meeting 2023, Lorraine Danks, Boitumelo Semete-Makokotlela, Sam Salek and Stuart Walker developed and presented a poster in which they shared the results, recommendations and [...]
A comparison of the Regional Medicines Regulatory Harmonisation Projects in East, West and Southern Africa.
During the DIA Global Annual Meeting 2023, Tariro Sithole, Nancy Ngum, Mercy Owusu-Asante, Stuart Walker and Sam Salek developed and presented a poster in which they shared the results, [...]
A comparison of regulatory decision patterns for oncology products to all other non-oncology products among Swissmedic, EMA and FDA
Consensus of regulatory decisions on the same Marketing Authorization Application (MAA) are critical for stakeholders. In this context, regulatory decision patterns from the Swissmedic (SMC), the US Food and [...]
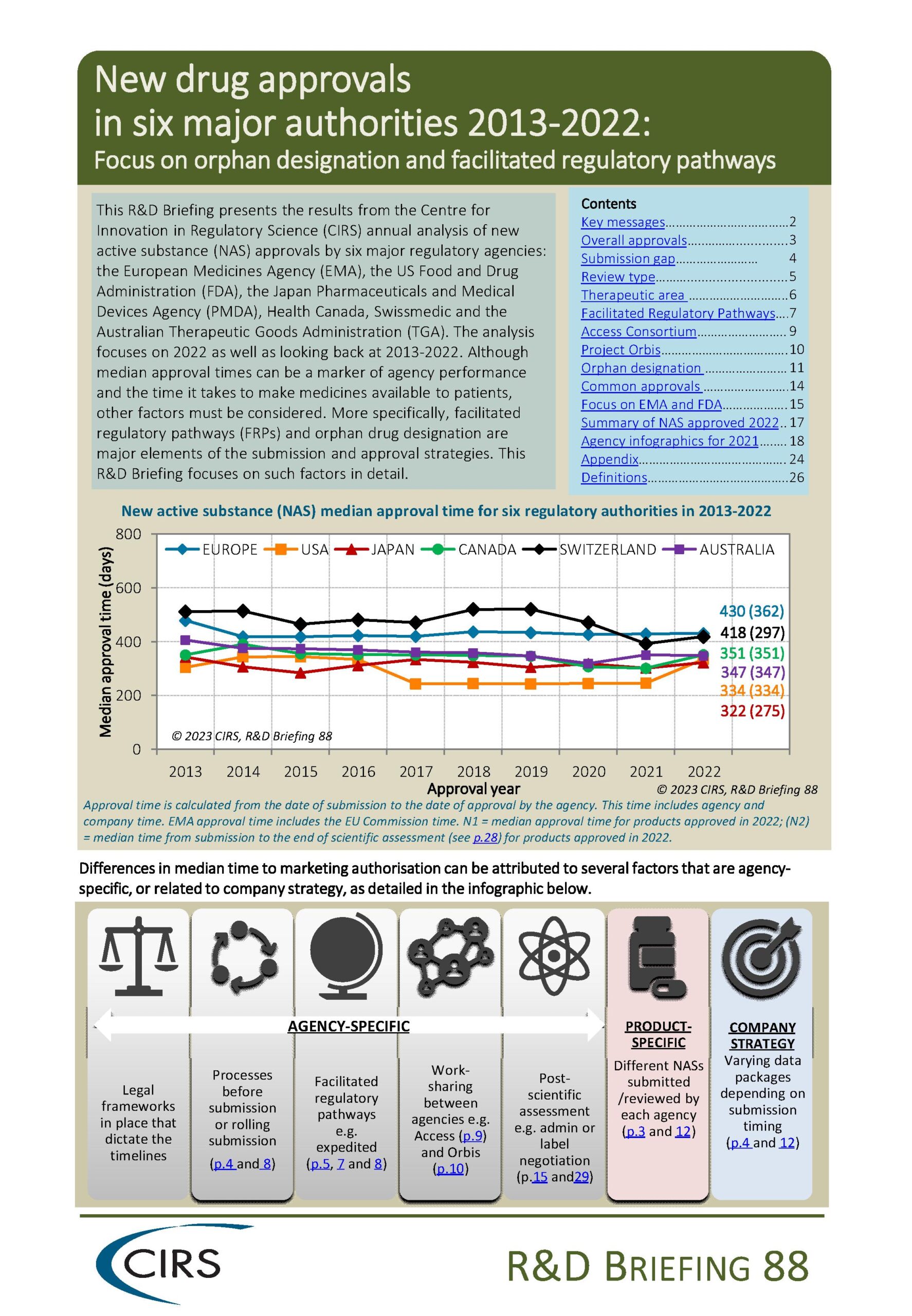
CIRS RD Briefing 88 – New drug approvals in six major authorities 2013-2022: Focus on orphan designation and facilitated regulatory pathways
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the [...]
CIRS RD Briefing 87 – A Roadmap for Regulatory Strengthening: CIRS Tools for Measuring and Optimising Regulatory Performance to Support Practices in Line with the World Health Organization Global Benchmarking Tool Indicators
Over the last 20 years, CIRS has been developing regulatory science tools to increase transparency of processes, support quality regulatory decision making, and provide global advocacy in support of [...]
Evaluation of Risk‑Based Approaches to the Registration of Medicines: Current Status Among African Regulatory Authorities
Background: Despite the worldwide need for increased access to safe and effective medicines, there is a lack of innovative medicines in many low- to middle-income countries. On the African [...]
Regulatory performance of the East African Community joint assessment procedure: The way forward for regulatory systems strengthening
Background: Seven national medicines regulatory authorities in the East African Community (EAC) have embraced regulatory reliance, harmonization and work sharing through the EAC Medicines Regulatory Harmonization programme. Measuring the [...]
Assessment of the effectiveness and efficiency of the West Africa medicines regulatory harmonization initiative by the pharma industry
Background: Following the establishment of Economic Community of West African States Medicines Regulatory Harmonization (ECOWAS-MRH) initiative in 2017, it was considered timely to carry out an evaluation of the current [...]
2022 Workshop report – Building on regulatory and HTA agilities for high unmet need
In 2020, coronavirus disease (COVID-19) spread rapidly around the world and halted regular social, business, and research activities. The pandemic impeded normal functioning across several fields, and businesses and [...]
2022 Workshop report – Collaborative models for regionalisation, work and information sharing: How do these fit into the regulatory toolkit?
This workshop looked at how maturing markets are building risk-based approaches into regulatory assessment, building on recent CIRS workshops in Singapore (2019) and South Africa (2018). The workshop also [...]
Regulatory, HTA and company interactions: the current landscape and future ecosystem for drug development, review and reimbursement
Background: Multi-stakeholder interactions have evolved at product and policy levels. There is a need to assess the current and future landscape of interactions between companies, and regulatory and HTA agencies [...]
CIRS 2022 Annual Report
We're delighted to present our latest Annual Report, which provides a summary of the projects, publications and Multi-stakeholder Workshops, Technical Fora, Industry Discussion Meetings, and Impact Case Studies from [...]
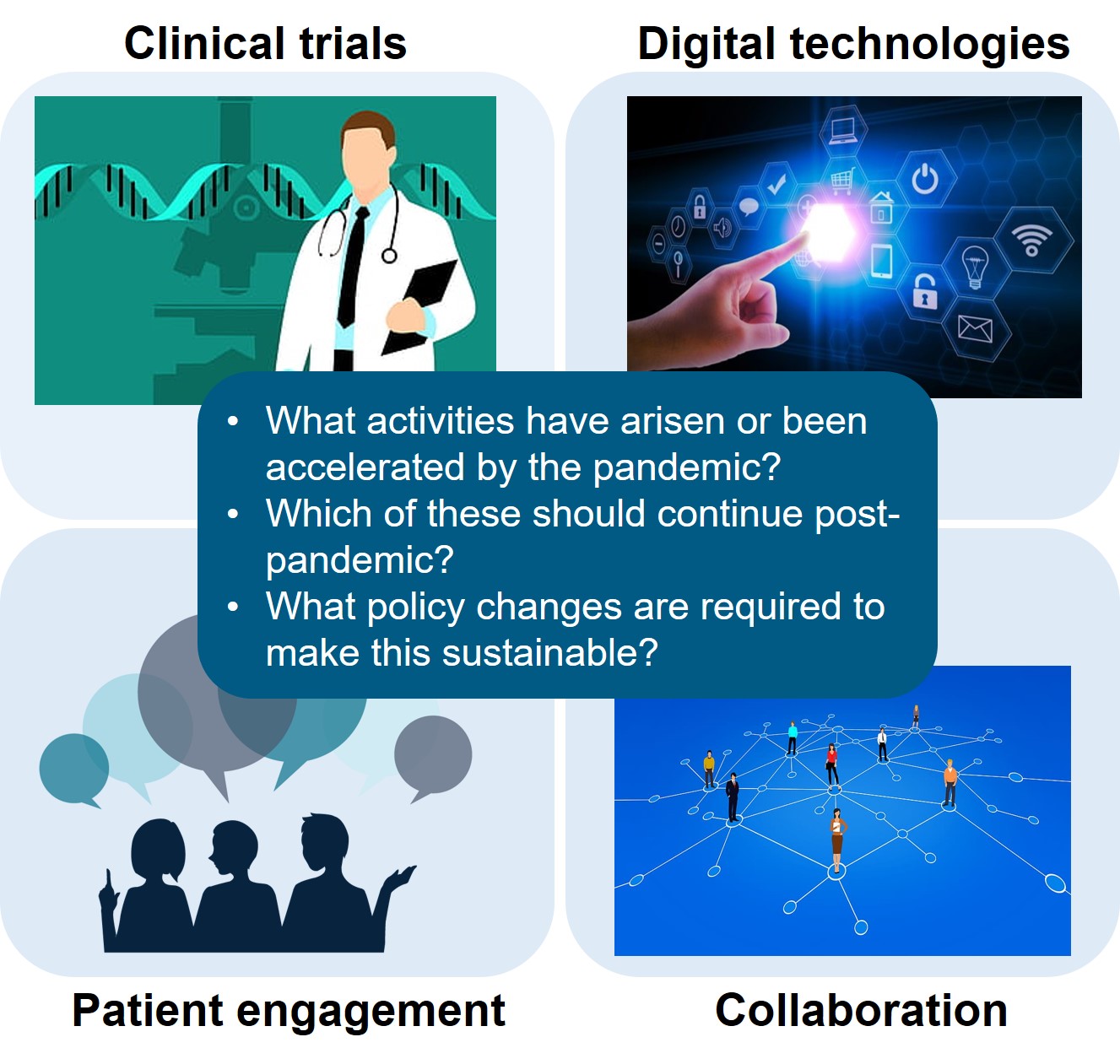
2022 Workshop report – How has the pandemic accelerated the acceptance and utility of RWD/RWE in regulatory/HTA decision making?
This workshop builds on the outcomes of the CIRS Professor Breckenridge memorial workshop in December 2020, as well as the 2021 workshop on utilisation of digital technologies in clinical [...]
Evolution of HTA practice and approaches from the perspectives of HTA agencies and pharmaceutical industry
Health technology assessment (HTA) has emerged as an important tool to support healthcare decision-makers to make rational reimbursement decisions, with the ultimate purpose of promoting an efficient healthcare system. [...]
2021 Workshop report – Regulatory and HTA landscape in Asia and Latin America
As regulatory systems mature with demand for new innovative treatments, jurisdictions are seeking to introduce more comprehensive healthcare systems; this is often accompanied by efforts to initiate health technology [...]
Assessment of the effectiveness and efficiency of the West Africa medicines regulatory harmonization initiative by the member countries
Background: The West Africa Health Organization launched the West Africa Medicines Regulatory Harmonization Project (WA-MRH) in 2017 with the overarching objective to improve the availability of high-quality, safe and [...]
Evaluation of the Food and Drugs Authority, Ghana Regulatory Review Process: Challenges and Opportunities
Purpose: This study aimed to assess the current regulatory review process of the food and drugs authority (FDA) Ghana by identifying key milestones, target timelines, good review practices and [...]
Evaluation of the East African Community joint assessment procedure by pharmaceutical companies
Background: A 2021 study to determine the viewpoints among the seven member countries regarding the effectiveness (i.e., achieving the intended outcomes) and efficiency (i.e., achieving the intended outcomes in [...]
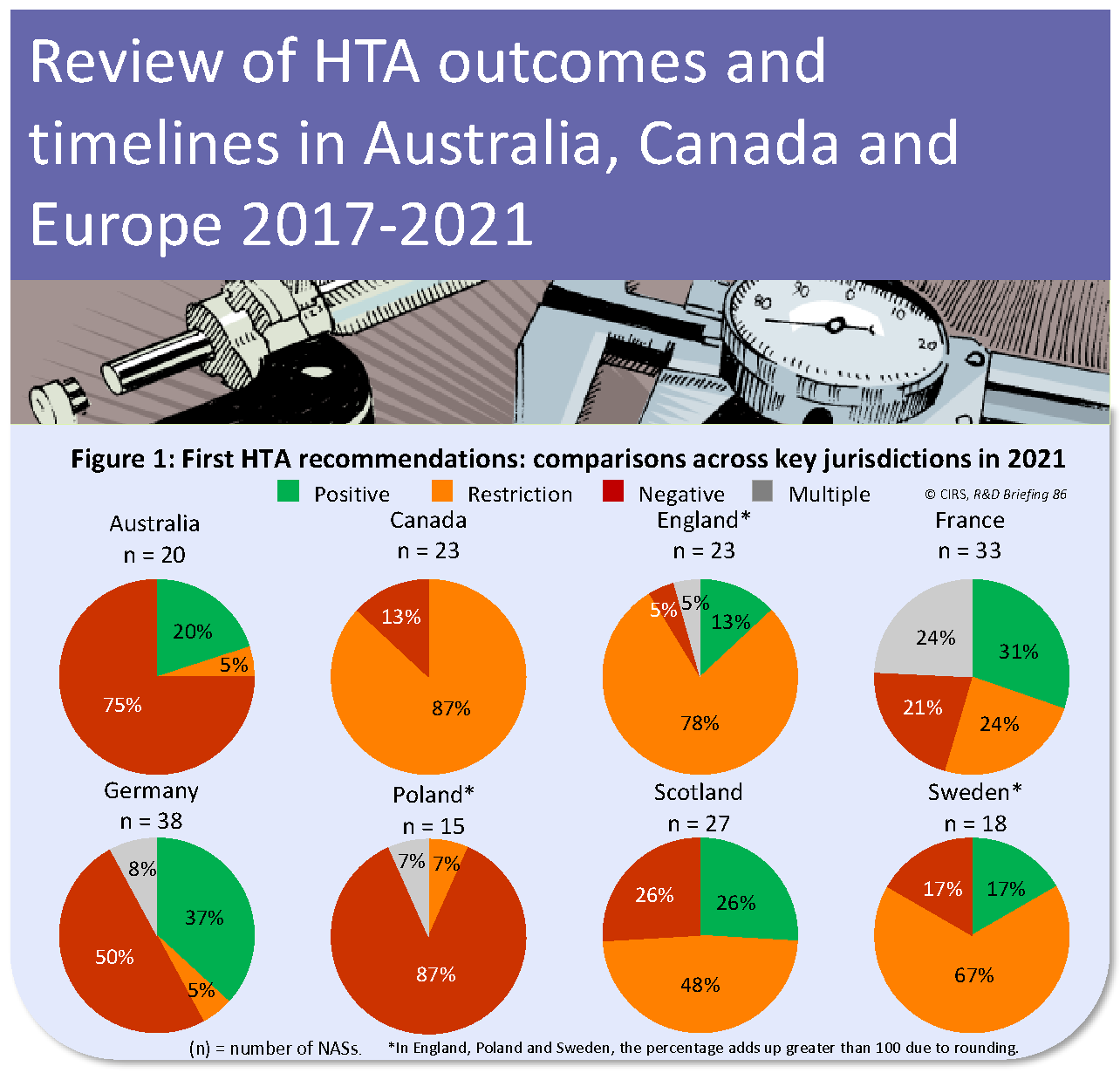
CIRS RD Briefing 86 – Review of HTA outcomes and timelines in Australia, Canada and Europe 2017-2021
The Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory [...]
Regulatory work-sharing initiative in Africa: ZaZiBoNa, past, present and future
PREFACE: The role of regulatory authorities in the health system is to ensure the quality, safety and efficacy of medical products. It is acknowledged that regulatory authorities are at [...]
Evaluation of the East African Community joint assessment
Background: For almost a decade, the East African Community has implemented the Medicines Regulatory Harmonization (EAC-MRH) programme among its member states to harmonise technical requirements and standards for medical [...]
Building HTA/Payer Perspectives Into Drug Development
Background: The target product profile (TPP) outlines the desired profile of a target product aimed at a particular disease and is used by companies to plan clinical development. Considering the [...]
Evaluation of the performance of the Gulf centralised registration procedure
Background: The Gulf Centralised Committee for Drug Registration (GCC-DR), as part of the Gulf Health Council (GHC), enables the consolidated registration of pharmaceutical products throughout the member states of [...]
CIRS RD Briefing 85 – New drug approvals in six major authorities 2012-2021
This Briefing presents the results from the CIRS annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food [...]
Industry evaluation of the efficiency and effectiveness of ZaZiBoNa
Introduction: The common technical document (CTD) format harmonised the requirements for the registration of medicines, which had traditionally differed from country to country, making it possible for countries to [...]
Regulatory evaluation of the efficiency and effectiveness of ZaZiBoNa
Introduction: ZaZiBoNa, the work-sharing initiative in the Southern African Development Community (SADC) that has been in operation for 8 years has successfully assessed over 300 dossiers/applications, with an overall [...]
CIRS RD Briefing 84 – China’s evolving regulatory landscape
China has made significant changes to its medicine regulatory system including: Regulatory reforms - since 2015, regulatory reforms have helped to eliminate application backlogs, improve review timelines and increase [...]
CIRS 2021 Annual Report
We're delighted to present our latest Annual Report, which provides a summary of CIRS projects, publications and workshops undertaken last year as well as case studies depicting CIRS’ impact [...]
2021 Workshop report – Digital technologies for clinical evidence generation
Digitisation and digital health technologies are transforming clinical development; companies, regulators and Health Technology Assessment (HTA) agencies are looking to derive actionable insights from the data being generated. This [...]
2021 Workshop report – Regulatory, HTA and payer interactions and collaborations
Over the last five years, regulatory and HTA interactions, as well multi-HTA and multi-regulatory interactions and collaborations, have evolved in thinking and mutual activities both at a product level [...]
2020 Workshop report – Reimagining regulatory models
As the regulatory landscape changes to meet new challenges, such as increasingly sophisticated medical innovations, fundamental questions are being raised: What is the role of a ‘modern’ regulator today? [...]
Comparison of the registration process of Zimbabwe with Australia, Canada, Singapore and Switzerland
Background: Benchmarking regulatory systems of low- and middle-income countries with mature systems provides an opportunity to identify gaps, enhance review quality, and reduce registration timelines, thereby improving patients’ access [...]
Seeking early scientific advice from HTA agencies
There is a growing trend for pharmaceutical companies to seek scientific advice on drug development from a Health Technology Assessment (HTA) perspective, to improve the efficiency of their studies, [...]
Regulatory-HTA decision-making interface: what the medical writer should know
For a new medicine to reach patients, it must achieve both regulatory marketing authorisation and reimbursement from the payer. Because regulators assess the benefits and risks of a medicine [...]
Review models and timelines in the Southern African Development Community
Introduction: Regulatory reliance, harmonization and work sharing have grown over the last few years, resulting in greater sharing of work and information among regulators, enabling efficient use of limited [...]
Good Review Practices in the Southern African Development Community
Introduction: National medicines regulatory agencies are faced with challenges including limited resources and technical capacity, resulting in countries collaborating and sharing resources to improve efficiency of the review process to [...]
2021 Project report – Monitoring implementation and adherence to ICH guidelines
Background: This study was built on the previous 2018/2019 assessment where ICH selected the Centre for Innovation in Regulatory Science (CIRS) to collaborate on the development and the conduct [...]
CIRS RD Briefing 83 – HTA outcomes in Australia, Canada and Europe 2016-2020
This Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory [...]
Keyter et al 2021 – Impact of reliance on South African review
Background: The aims of this study were to compare the overall regulatory review timelines achieved by the South African Health Products Regulatory Authority (SAHPRA) in 2020 to the timelines historically [...]
2020 Workshop report – Effectiveness of the regulatory approval process
This workshop was part of a series of global development workshops that brought together mature and maturing regulatory agencies. These workshops successively built on one another and evolved from [...]
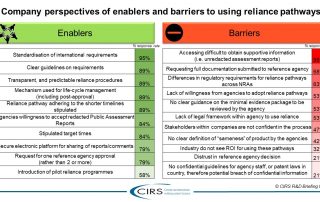
CIRS RD Briefing 82 – Regulatory reliance pathways: opportunities and barriers
An increasing number of National Regulatory Authorities (NRAs) are turning to reliance as a way to conserve resources, build expertise and capacity, increase the quality of their regulatory decisions, [...]
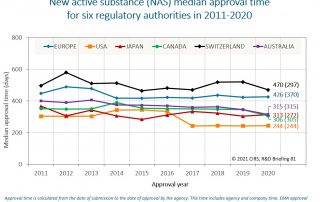
CIRS RD Briefing 81 – New drug approvals in six major authorities 2011-2020
This Briefing presents the results from the CIRS annual analysis of New Active Substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food [...]
CIRS 2020 Annual Report
We're delighted to present our latest Annual Report, which provides a summary of CIRS projects and workshops undertaken in 2020 as well as case studies depicting CIRS’ impact on [...]
Bujar et al 2021 – Transparency in EMA and FDA decision making
Although it cannot be expected that different medicines’ regulatory agencies always reach the same review outcome, it is important that decision making is documented and communicated to ensure transparency. [...]
CIRS RD Briefing 80 – Reimagining medicine regulatory models
This R&D Briefing summarises the outputs of breakout group discussions held during a CIRS multi-stakeholder workshop in December 2020 entitled ‘Reimagining medicine regulatory models: implementing fit-for-purpose sustainable activities for [...]
CIRS RD Briefing 79 – Use of advisory committees in Colombia
This Briefing provides an overview of how advisory committees can be used to support the regulatory decision-making process and considering the context of Latin American regulatory systems, aims to [...]
Bujar et al 2021 – Value of Facilitated Regulatory Pathways
Background: Despite the growing application of facilitated regulatory pathways (FRPs), little attention has focused on assessing the perception of pharmaceutical companies regarding their usefulness beyond increasing timeliness. Objectives: The [...]
Sithole et al 2021 – Regulatory review process in Zimbabwe
Purpose: The aims of this study were to assess the current regulatory review process of the Medicines Control Authority of Zimbabwe (MCAZ), identify key milestones and target timelines, evaluate [...]
Wang et al 2020 – Companies’ HTA strategies and practices
Background: Health technology assessment (HTA) has increased in importance in supporting payer decision making by assessing the relative effectiveness and cost effectiveness of new medicines. Thus, pharmaceutical companies need to [...]
Keyter et al 2020 – A proposed regulatory review model to support SAHPRA
Background: National regulatory agencies of various sizes and maturity levels, including the South African Health Products Regulatory Authority (SAHPRA), have had to revise systems and re-engineer processes in order [...]
2019 Workshop report – Optimising the regulatory review process by evaluating performance and addressing good reliance practices
This workshop was part of a series of workshops on reliance, which continues to be an important area of focus for CIRS. It builds on the outcomes of a [...]
2019 Workshop report – Identifying and understanding uncertainty during development
This workshop was a follow-on from a collaborative forum held in Utrecht in 2018 entitled What new research can enable a joint approach by regulatory and HTA agencies to [...]
Liberti et al 2020 – Evaluation of the Caribbean Regulatory System centralised assessment process
Background: The Caribbean Regulatory System is a centralized medicine assessment procedure established to serve the needs of the Member States of the CARICOM region. In order to better understand [...]
Wang et al 2020 – Benchmarking HTA agencies: methodological challenges and recommendations
Objectives: The objectives of the study were to establish a benchmarking tool to collect metrics to enable increased clarity regarding the differences and similarities across health technology assessment (HTA) [...]
Roadmap for Regulatory Performance
Every regulatory authority in the world has an ambition to improve its performance. In order to achieve this, agencies often establish performance indicators which they use for measuring and [...]
CIRS 2019 Annual Report
We're delighted to present the inaugural CIRS Annual Report, which provides a summary of projects and workshops undertaken in 2019, as well as a historical perspective of CIRS achievements [...]
An independent perspective on the East African Community Medicines Regulatory Harmonisation initiative
African regulators are taking bold transformative steps to optimise the effective and efficient use of their agency resources to assure access to quality, safe and effective medicines. In particular, [...]
CIRS RD Briefing 78 – HTA outcomes in Australia, Canada and Europe 2015-2019
This Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory [...]
Bujar et al 2020 – A Process for Evaluating Quality Decision-Making Practices
Background: The development of a medicine is not only underpinned by good science but also by Quality Decision-Making Practices (QDMPs). Indeed, it is important to ensure that all organisations [...]
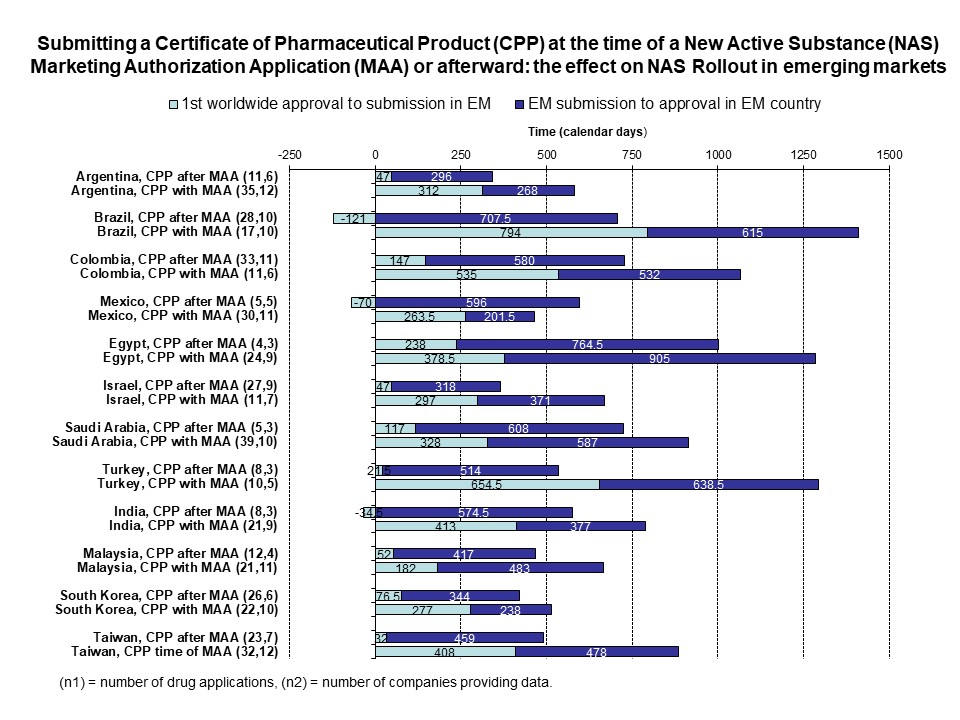
Rodier et al 2020 – Use of the CPP in 18 maturing markets
Background: The certificate of pharmaceutical product (CPP) was implemented to accelerate the availability of new drugs in developing countries by providing evidence of the quality of products and reducing [...]
Keyter et al 2020 – Can standardisation of the Public Assessment Report improve benefit-risk communication?
Background: National regulatory authorities (NRAs) make the decision to register a medicine based on an assessment of its benefits and risks and publicly available assessment reports are used as [...]
Bujar et al 2020 – Documentation of Quality Decision-Making Practices
Background: Pharmaceutical companies and regulatory agencies endeavor to relate their decision making with outcomes to improve future decision making and to ensure that gained knowledge is fed back into [...]
Patel et al 2020 – Analysis of regulatory review timelines for ANVISA
Background: The Brazilian health regulatory agency (Agência Nacional de Vigilância Sanitária, ANVISA) has embarked on transformational initiatives to fulfill its mandate to provide timely access to safe, effective, and [...]
CIRS RD Briefing 77 – New drug approvals in six major authorities
This Briefing presents the results from the CIRS annual analysis of New Active Substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food [...]
Mohd Sani et al 2020 – Evaluation of the Malaysian regulatory process using OpERA methodology
Introduction: The National Pharmaceutical Regulatory Agency (NPRA) embarked on a regulatory-strengthening program and is evaluating its processes. Optimising Efficiencies in Regulatory Agencies (OpERA) is a regulatory-strengthening program that provides [...]
Sithole et al 2020 – Evaluating the success of ZaZiBoNa
The Southern African Development Community (SADC) collaborative medicines registration initiative ZaZiBoNa is a successful regional work-sharing initiative on the African continent. This paper reviews the history of the ZaZiBoNa [...]
CIRS RD Briefing 76 – Mexican therapeutic landscape
An efficient regulatory process can be reflected in measurable positive health impacts; conversely, activities that slow or impede regulatory efficiency and predictability can be detrimental. Recent developments in the [...]
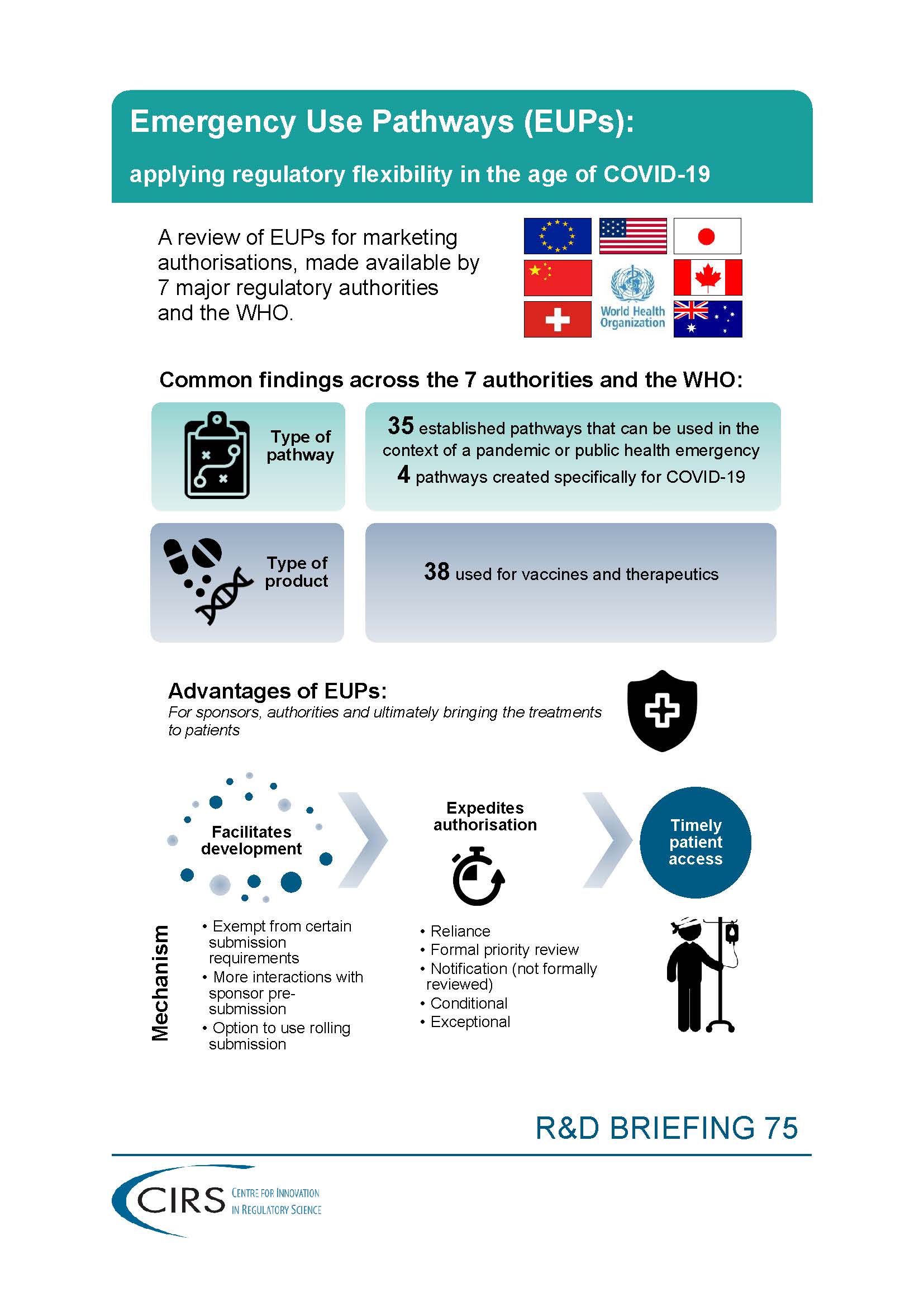
CIRS RD Briefing 75 – Emergency Use Pathways (EUPs)
It has become clear that agencies have a number of pathways that can be used during public health emergencies for the authorisation of therapeutics and vaccines. Some of these [...]
Keyter et al 2020 – Implementation of a Framework for an Abridged Review Using Good Reliance Practices: Optimising the Medicine Regulatory Review Process in South Africa
Background This study sought to identify criteria and current practices for implementing an abridged review process and understanding barriers and enablers in utilizing reliance models and to offer recommendations [...]
Mashaki Ceyhan et al 2020 – Patients’ perspectives of the pharmaceutical regulatory and reimbursement system in Istanbul, Turkey
The aim of this study was to explore patients’ knowledge and perspectives in Istanbul, Turkey about the pharmaceutical regulatory review and reimbursement processes with respect to access to new [...]
Koyuncu et al 2020 – Evaluation of the performance of the Turkish regulatory agency: recommendations for improved patients’ access to medicines
Background: This study was to evaluate the Turkish regulatory review process and timelines between 2016 and 2018 with a view to assess the changes that had taken place since [...]
CIRS RD Briefing 74 – OpERA programme
CIRS has collected regulatory assessment data for over 20 years, initially with ICH and ICH-observing countries. The OpERA programme, “Optimising Efficiencies in Regulatory Agencies (OpERA)”, was initiated through CIRS [...]
December 2019 Slide of the Month
December's Slide of the Month is taken from a CIRS poster recently presented at ISPOR Europe 2019. Does FDA Breakthrough Designation affect HTA recommendation in terms of timing and outcome? [...]
Keyter et al 2019 – Evaluation of the performance of the South African regulatory agency
Background: Timely access to new medicines may be addressed through strengthening of registration efciencies and timelines by establishing and refning value-added registration processes, resources, and systems. The aims of [...]
Kühler et al 2019 – To what degree are review outcomes aligned for new active substances between the EMA and the US FDA?
Objective To compare review outcome alignment between European Medicines Agency (EMA) and US Food and Drug Administration (FDA) for medicines approved by both agencies in the time period 2014–2016. Design [...]
Project report – Monitoring implementation and adherence to ICH guidelines
Background: According to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Articles of Association, the regulatory members of ICH are expected to implement [...]
Executive Colloquium report – What is the value and return on investment for our company to maintain a regulatory policy function?
In June 2019, the Centre for Innovation in Regulatory Science (CIRS) held an Executive Colloquium in Rockville, MD, USA that brought together representatives from multinational pharmaceutical companies to gauge [...]
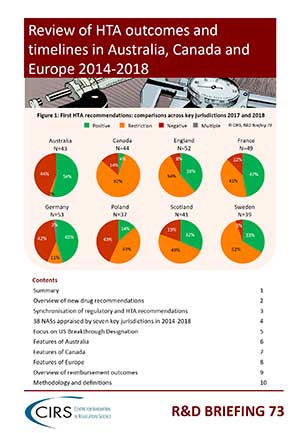
CIRS RD Briefing 73 – HTA outcomes 2014-18
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure that patient access to medicines of therapeutic value is not delayed. As part of an [...]
CIRS RD Briefing 71 – Trends in the regulatory landscape Latin America
To address the complex challenges in the global regulatory environment and the growing demand for patient access to new medicines, regulatory agencies in Latin America are actively engaging in regulatorystrengthening [...]
CIRS RD Briefing 72 – Trends in the regulatory landscape Asia
To address the complex challenges in the global regulatory environment and the growing demand for patient access to new medicines, regulatory agencies in Asia are actively engaging in regulatory-strengthening and [...]
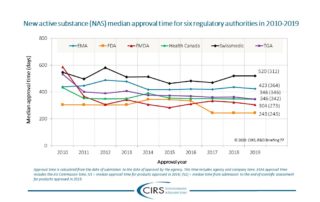
CIRS RD Briefing 70 – New approvals in six regulatory authorities 2009-18
Major improvements in the regulatory environment as well as changes in strategies of multinational companies have led to a general decrease in the time to marketing authorisation and improved consistency [...]
McAuslane et al 2019 – Confluence of Accelerated Regulatory and HTA Access Pathways
There is a growing interest in aligning accelerated regulatory pathways with flexible access and reimbursement pathways to expedite the equitable availability of high-quality, safe, and effective medicines that provide [...]
Keyter et al 2019 – South African Medicines Control Council: Comparison of Its Registration Process
Introduction: Comparisons between regulatory authorities of similar size and regulatory characteristics facilitate value-added benchmarking and provide insight into regulatory performance. Such comparisons highlight areas for improvement as authorities move [...]
Bujar et al 2019 – Quality decision making in Health Technology Assessment
Background: To evaluate the quality of the decision-making processes of pharmaceutical companies during medicines development for evidence generation to support reimbursement of new medicines and the appraisal recommendation decision-making process [...]
Bujar et al 2019 – Reliability and relevance of a decision-making instrument
Introduction: The Quality of Decision-Making Orientation Scheme (QoDoS) was developed to provide organisations involved in submission, approval and reimbursement of new medicines with a tool to improve the quality [...]
2018 Forum report – Managing uncertainties for products using early access pathways
In November 2018, a collaborative forum presented by CIRS and the Utrecht University WHO Collaborating Centre for Pharmaceutical Policy and Regulation brought together regulatory, HTA, industry and academic perspectives [...]
2017 Workshop report – Flexible regulatory/access pathways
The aim of this workshop was to bring together companies, patient representatives and HTA, payer and regulatory agencies to discuss current perspectives and opportunities for Facilitated Regulatory Pathways (FRPs) [...]
CIRS RD Briefing 69: Review of HTA outcomes and timelines 2014-2017
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure that patient access to medicines of therapeutic value is not delayed. As part of [...]
CIRS RD Briefing 68: Regulatory and HTA decision making and access to medicines – the consequences of sequence
Historically, every jurisdiction with some form of regulatory agency capacity has undertaken the review of medicines as a first step in the market access process. This step is intended [...]
Wang et al 2018 – Building Synergy between Regulatory and HTA Agencies
Objectives: To evaluate the current practice of companies and agencies to assess the changes made in aligning regulatory and health technology assessment (HTA) stakeholders; to identify areas of commonality [...]
2018 Workshop report – Practical implementation of reliance models
This Workshop was a follow-on from that held in 2017 in Sao Paulo entitled “Facilitating the review of new medicines through risk-based evaluations: How can a stratification process be [...]
CIRS RD Briefing 67 – New approvals in six regulatory authorities 2008-2017
Major improvements in the regulatory environment as well as changes in strategies of multinational companies have led to a decrease in the time to marketing authorisation as well as [...]
CIRS RD Briefing 66 – Benefit-Risk Assessment Tool (BRAT)
A structured approach to benefit-risk assessment is required as the cornerstone of a consistent way to evaluate and communicate observations regarding a medicine’s benefit-risk profile. The Benefit Risk Action Team [...]
CIRS RD Briefing 65 – New approvals in six regulatory authorities 2007-2016
Over the last decade, 2007-2016, convergence in approval times as well as changes in strategies of multinational pharmaceutical companies have resulted in more new active substances (NASs) being internationalised, [...]
Quality decision-making practices
This book compiles the presentations and syndicate discussions from a CIRS workshop that brought together representatives from international pharmaceutical companiesand regulatory authorities from Europe, US, Canada and Australia and [...]
CIRS RD Briefing 64: Review of HTA outcomes and timelines 2014-2015
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure patient access to medicines of therapeutic value. As part of an ongoing study to [...]
CIRS RD Briefing 63: HTA process maps
This R&D Briefing summarises the background and methodology of process mapping with examples to demonstrate the steps involved in regulatory, HTA and coverage processes for new medicines. Process maps [...]
2017 Workshop report – Facilitating review through risk-based evaluations
This Workshop built on previous CIRS global development workshops as well the work being undertaken by various groups in the areas of good regulatory and review practices and focused [...]
Globally Applicable Facilitated Regulatory Pathways to Improve Equitable Access to Medicines
A variety of approaches have been developed to accelerate the regulatory review of medicines. We characterise these various expedited pathways as facilitated regulatory pathways (FRPs): regulatory pathways designed to accelerate [...]
CIRS RD Briefing 62 – New drug approvals in ICH countries 2007-2016
There have been major improvements in the regulatory environment in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) countries over the [...]
CIRS RD Briefing 61: Building quality into decision-making processes
In 2015, CIRS initiated a programme in Quality Decision Making with the following aims: Evaluate the current decision-frameworks and understand the characteristics of different decision-making processes Assess the quality [...]
Haqaish et al 2017 – Jordan Food and Drug Administration: Comparison of its Registration Process with Australia, Canada, Saudi Arabia and Singapore
Objective: This study compares the current regulatory review process and good review practices at the Saudi Food and Drug Authority (SFDA) with those of regulatory agencies in Australia, Canada, [...]
2016 Workshop report – Commonality in evidentiary requirements
Commonality in evidentiary requirements across regulatory and HTA stakeholders 21-22 SEPTEMBER 2016, SURREY, UK Workshop objectives Discuss the progress made to align evidentiary requirements, what the drivers have been [...]
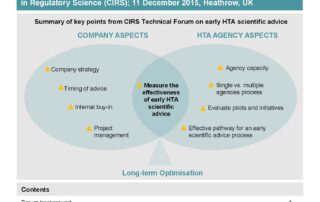
CIRS RD Briefing 60 – Early scientific advice from HTA agencies
This R&D Briefing 60 summarises highlights from the Technical Forum convened by CIRS on 11 December 2015, Heathrow, UK. Forum objectives: Identify companies’ current approaches to seeking early scientific [...]
2016 Workshop report – RWD to RWE for assessing efficacy and effectiveness
Real-world data to real-world evidence for assessing efficacy and effectiveness: Opportunities and challenges for new medicines development, regulatory review and health technology assessment 23-24 JUNE 2016 TYSONS CORNER, VIRGINIA, [...]
CIRS RD Briefing 59 – New approvals in six regulatory authorities 2006-2015
The last decade, 2006-2015, has seen a continuation of the convergence and general decrease in the approval times amongst six major regulatory authorities, namely the European Medicines Agency (EMA), [...]
2016 Workshop report – Key performance metrics for regulatory processes
WHAT ARE THE KEY PERFORMANCE METRICS THAT AGENCIES AND COMPANIES SHOULD USE TO MEASURE REGULATORY PROCESSES AND PRACTICES TO FACILITATE THE LICENSING OF NEW MEDICINES? 3-4 FEBRUARY 2016, KUALA [...]
Hashan et al 2016 – Saudi Arabia FDA: An Evaluation of the Registration Process and Good Review Practices in Saudi Arabia in Comparison with Australia, Canada and Singapore
Objective: This study compares the current regulatory review process and good review practices at the Saudi Food and Drug Authority (SFDA) with those of regulatory agencies in Australia, Canada, [...]
Al-Rubaie et al 2015 – Agency and industry views of the efficiency of the Gulf centralised registration procedure
The aim of this study was to examine the views and experiences of the Gulf Cooperation Council (GCC) states and pharmaceutical companies to identify the strengths and weaknesses of [...]
CIRS RD Briefing 58: Changing regulatory environment in Latin America
The aim of this Briefing is to review and summarise the findings from the major studies and interactions carried out by CIRS in LATAM in the last decade in [...]
2015 Workshop report – Patient involvement in review and reimbursement
What is the patient’s role in informing the decision process for approval and reimbursement of new medicines? 7-th October 2015, Windsor, UK This workshop built on CIRS workshops on [...]
CIRS RD Briefing 57 – New drug approvals in ICH countries 2005-2014
There have been major improvements in the regulatory environment in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) countries over the [...]
CIRS RD Briefing 56: Understanding the dynamics of China’s regulatory environment
Changes have occurred in the organisation and procedural activities of the China Food and Drug Administration (CFDA) and the Centre for Drug Evaluation (CDE). Initiatives that were designed to [...]
Walker et al 2015 – Universal Framework for the Benefit-Risk Assessment of Medicines
A universal framework for the evaluation of the benefit-risk assessment of medicines during development by pharmaceutical companies and in the regulatory review by regulatory authorities is considered of value, [...]
Al-Rubaie et al 2014 – Evaluation of the Gulf centralised registration procedure
The aim of the study was to evaluate the Gulf Cooperation Council (GCC) centralized regulatory review process. Regulatory review times—including submission and application dates for new active substances (NASs) [...]
CIRS RD Briefing 55 – Approvals across six major authorities 2004-2013
As part of the ongoing study to monitor regulatory performance, CIRS has analysed the trends in new medicines’ approval between 2004 and 2013 by six regulatory authorities including Health [...]
2013 Workshop report – Commonality across decision frameworks used by HTA and regulatory agencies
IS THERE A COMMONALITY ACROSS THE STRUCTURED DECISION FRAMEWORKS USED BY HTA AND REGULATORY AGENCIES? 1st-2nd October 2013, Surrey, UK This Workshop was designed to bring together the various [...]
CIRS RD Briefing 54 – Approvals in ICH countries 2004-2013
In 2013, the overall number of New Active Substances (NASs) approved by EMA, FDA and PMDA was comparable across the three agencies. Nevertheless, despite this similarity, the number of [...]
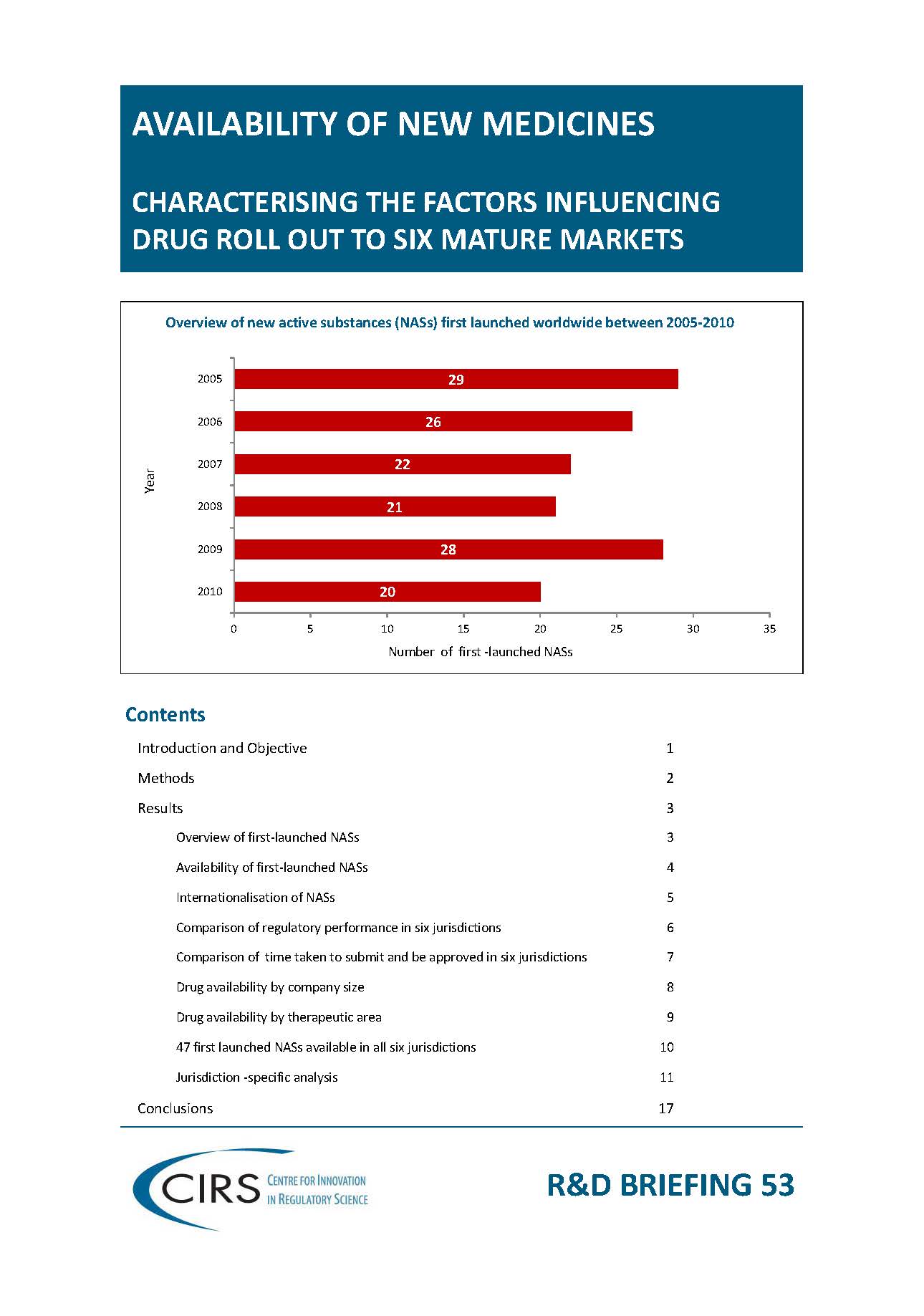
CIRS RD Briefing 53 – Factors influencing drug roll out to six mature markets
Objective: To review NASs first launched between 2005‐2010 and to determine their regulatory status as of 31 December 2012 in USA, Europe, Japan, Canada, Switzerland and Australia to identify [...]
Liu et al 2013 – Characterising Good Review Practices across APEC agencies
As a first step in the implementation of the Asia-Pacific Economic Cooperation (APEC) Best Regulatory Practice Project, the Centre for Innovation in Regulatory Science conducted a gap analysis survey [...]
Allen et al 2013 – Archetypes for non-ranking classification and comparison of European HTA systems
Introduction: European countries are increasingly utilising health technology assessment (HTA) to inform reimbursement decision-making. However, the current European HTA environment is very diverse, and projects are already underway to [...]
CIRS RD Briefing 52 – New drug approvals in ICH countries 2003-2012
Active Substances (NASs) approved by both the FDA and PMDA represented the largest number of new medicines approved this decade. Regulatory approvals by EMA were lower than the other [...]
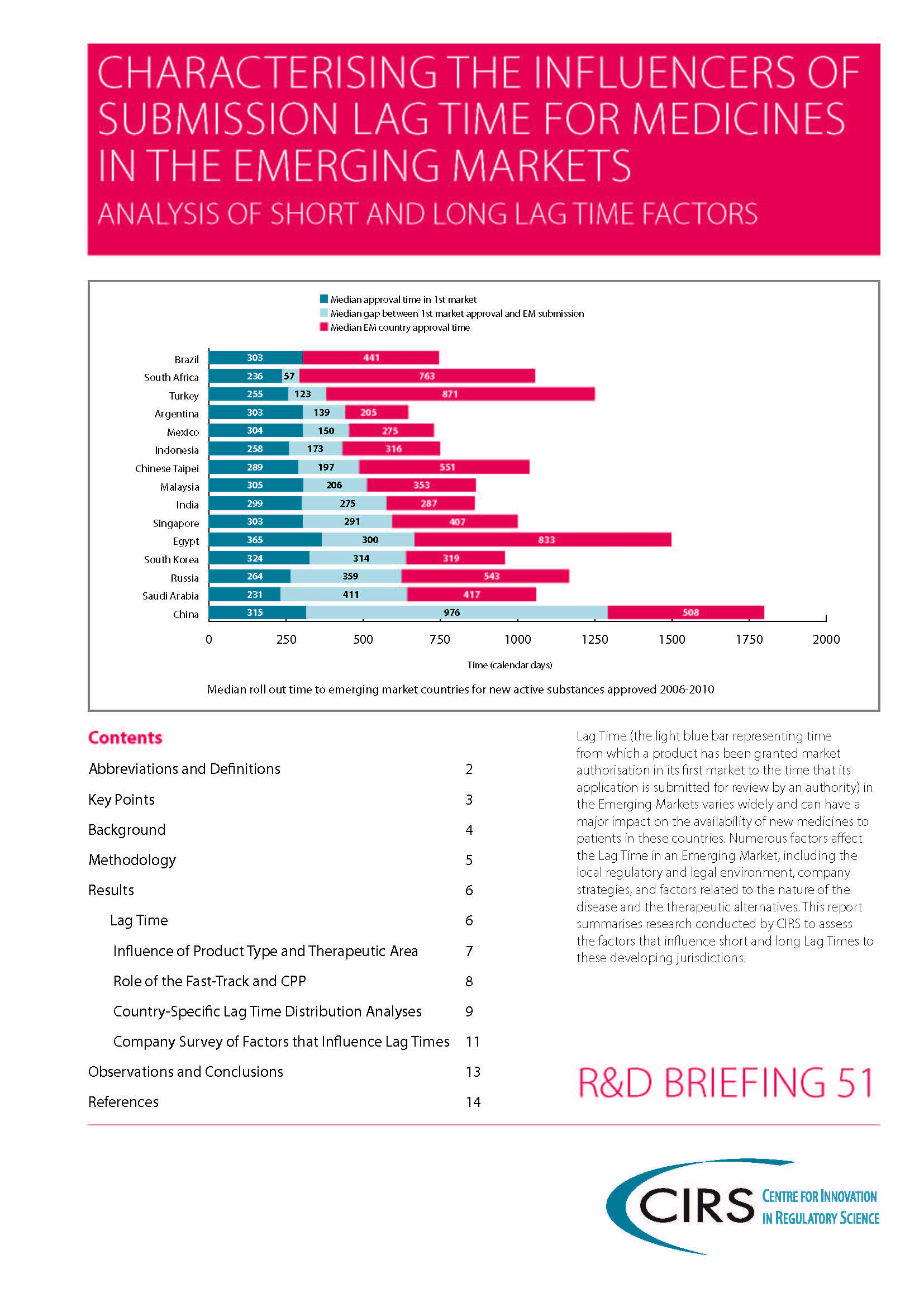
CIRS RD Briefing 51 – Submission lag time in the emerging markets
Lag Time (the time from which a product has been granted market authorisation in its first market to the time that its application is submitted for review by an [...]
CIRS RD Briefing 51 – New drug approvals in ICH countries 2002-2011
In 2011, the number of New Active Substances (NASs) approved in all the ICH countries increased compared to 2010, and FDA represented the largest number of new medicines approved [...]
Salek et al 2012 – Scorecards to Assess the Quality of a Regulatory Submission and Its Review
An efficient review depends not only on timely approval but also on ensuring the quality of the process from construction of the dossier to the ultimate regulatory decision. Two [...]
Liberti et al 2010 – Progress on the development of a benefit-risk framework for evaluating medicines
CIRS authors write about benefit-risk in the March 2010 issue of Regulatory Focus by the Regulatory Affairs Professionals Society (RAPS). Article reproduced with the permission of RAPS. Download [...]
Hirako et al 2007 – Comparison of the drug review process at five international regulatory agencies
Regulatory approval time is a key metric that is used to evaluate the performance of regulatory agencies. A new methodology has been developed to compare the regulatory review process [...]
CIRS RD Briefing 46: Building quality into regulatory activities
The Institute for Regulatory Science is currently involved in several activities related to quality as it applies to regulatory submissions and procedures, rather than the more conventional association with [...]
CIRS RD Briefing 50 – Cross-regional comparison of regulatory environment in emerging markets
The emerging markets of Asia-Pacific, the Middle East, Africa and Latin America are becoming increasingly important to pharmaceutical companies in their global strategies for the registration of new medicines [...]
CIRS RD Briefing 49 – Accessing the regulatory environment – Latin America
The Regulatory Agencies in Latin America share a common goal with the research-based pharmaceutical companies that they regulate. This is to ensure that new medicines become available to patients [...]
CIRS RD Briefing 48 – Assessing the regulatory environment – Middle East and Africa
The regulatory agencies in the Middle East and African region that were included in this study share a common goal with the research-based pharmaceutical companies that they regulate. This [...]
CIRS RD Briefing 47 – Accessing the regulatory environment – SE Asia and Western Pacific
The regulatory agencies in South East Asia and the Western Pacific share a common goal with the research-based pharmaceutical companies that they regulate. This is to ensure that new [...]
CIRS RD Briefing 44: Global drug development and regulatory review
Although it is the goal of most major pharmaceutical companies to achieve global development for new medicines with simultaneous submission of the regulatory dossier in the three main ICH [...]
CIRS RD Briefing 43: Current strategies in global drug development
A summary of the outcome of a survey carried out by the CMR International Institute for Regulatory Science among pharmaceutical companies. Although the pharmaceutical industry is moving towards integrated [...]
CIRS RD Briefing 45: Regulatory timelines in Saudi Arabia
This unique study is the first in-depth analysis of the trends and changes in the regulatory approval times for medicines in the Kingdom of Saudi Arabia. Not only have [...]
CIRS RD Briefing 42: Changing regulatory environment
A summary of the outcome of a survey carried out by the CMR International Institute for Regulatory Science among pharmaceutical companies and regulatory agencies. There was consensus that the [...]
CIRS RD Briefing 41 – Workshop on Regulatory Performance
Highlights from the workshop entitled 'Regulatory Performance: Critical Success Factors in Today’s Environment', held by the CMR International Institute for Regulatory Science in Washington D.C., 15-16 September 2003 In [...]
CIRS RD Briefing 40 – Pharmacogenetics and pharmacogenomics in drug development
Summarised results of a survey carried out among pharmaceutical companies and regulatory agencies, by the CMR International Institute for Regulatory Science Pharmacogenetic (PGt) and pharmacogenomic (PGx) technologies are being [...]
CIRS RD Briefing 39 – Regulating personalised medicine
Highlights of the workshop "Regulating personalised medicine" organised by the CMR International Institute for Regulatory Science, Nutfield Priory, Surrey, UK, 14-15 April 2003 The unravelling of the human genome [...]
CIRS RD Briefing 38 – Risk management
Highlights of the CMR International institute Workshop on Risk Management: The role of regulatory strategies in the development of new medicines, Washington DC, 12-13 December 2002 Following a number [...]
CIRS RD Briefing 37 – Adoption of ICH E5 in Asia Pacific
In 2001, CMR International conducted a study among pharmaceutical companies to evaluate their experience with regulatory authorities in Asia Pacific regarding the acceptance of foreign clinical data and adoption [...]
CIRS RD Briefing 36 – Impact of ICH E5 guideline
The ICH E5 Guideline was introduced in February 1998 and subsequently implemented by the regulatory authorities of the USA, EU and Japan. The purpose of this guideline is to [...]
CIRS RD Briefing 31 – Regulatory review times
CMR International R&D Briefing 31 - Profile of performance: Review times - is there still room for improvement? Key messages: Performance improvement initiatives established by regulatory authorities in the [...]
CIRS RD Briefing 97 – Access Consortium and Project Orbis Approvals Across Eight Regulators
This R&D Briefing builds upon the Centre for Innovation in Regulatory Science (CIRS)'s long-standing efforts to examine trends and practices in regulatory approvals. For over 20 years, CIRS has [...]
CIRS RD Briefing 98 – European HTA trends: HTA outcomes and timelines across seven markets 2019-2023
This R&D Briefing presents data from HTADock, an ongoing CIRS metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies. It [...]
CIRS RD Briefing 96 – Review of HTA outcomes and timelines in Australia, Canada and the UK 2019-2023
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies. It focuses [...]
CIRS RD Briefing 95 – Review of HTA outcomes and timelines in Australia, Canada, Europe and the UK 2019-2023
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies, each with [...]
CIRS RD Briefing 93 – New drug approvals by six major authorities 2014-2023
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the [...]
CIRS RD Briefing 94 – Value of Reference Agency Reports in Enabling Reliance
Access to information, including the assessment documents of reference national regulatory agencies (NRA), is a key enabler of regulatory risk-based decision making. It promotes an understanding of what was [...]
CIRS RD Briefing 92 – Appraising the usability of public assessment reports for reliance
Regulatory reliance facilitates regulatory approvals, allows the use of resources more efficiently, and ultimately serves patients by accelerating access to quality-assured, safe, and effective medicines. The World Health Organisation [...]
CIRS RD Briefing 91 – Approaches to Implementing Regulatory Reliance: Considerations for Agencies
This CIRS briefing delves into the increasingly pivotal role of regulatory reliance in the global pharmaceutical landscape. Reliance is defined by World Health Organization (WHO) as the act whereby [...]
CIRS RD Briefing 90 – Challenges and opportunities for orphan medicines availability in Mexico
The document ‘Estrategia sobre Certidumbre Regulatoria para el Sector Farmacéutico’ (Strategy of Regulatory Certitude for the Pharmaceutical Sector), published last January by COFEPRIS describes important working projects the agency [...]
CIRS RD Briefing 89 – Review of HTA outcomes and timelines in Australia, Canada, Europe and the UK 2018-2022
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies, each with [...]
CIRS RD Briefing 88 – New drug approvals in six major authorities 2013-2022: Focus on orphan designation and facilitated regulatory pathways
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the [...]
CIRS RD Briefing 87 – A Roadmap for Regulatory Strengthening: CIRS Tools for Measuring and Optimising Regulatory Performance to Support Practices in Line with the World Health Organization Global Benchmarking Tool Indicators
Over the last 20 years, CIRS has been developing regulatory science tools to increase transparency of processes, support quality regulatory decision making, and provide global advocacy in support of [...]
CIRS RD Briefing 86 – Review of HTA outcomes and timelines in Australia, Canada and Europe 2017-2021
The Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory [...]
CIRS RD Briefing 85 – New drug approvals in six major authorities 2012-2021
This Briefing presents the results from the CIRS annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food [...]
CIRS RD Briefing 84 – China’s evolving regulatory landscape
China has made significant changes to its medicine regulatory system including: Regulatory reforms - since 2015, regulatory reforms have helped to eliminate application backlogs, improve review timelines and increase [...]
CIRS RD Briefing 83 – HTA outcomes in Australia, Canada and Europe 2016-2020
This Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory [...]
CIRS RD Briefing 82 – Regulatory reliance pathways: opportunities and barriers
An increasing number of National Regulatory Authorities (NRAs) are turning to reliance as a way to conserve resources, build expertise and capacity, increase the quality of their regulatory decisions, [...]
CIRS RD Briefing 81 – New drug approvals in six major authorities 2011-2020
This Briefing presents the results from the CIRS annual analysis of New Active Substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food [...]
CIRS RD Briefing 80 – Reimagining medicine regulatory models
This R&D Briefing summarises the outputs of breakout group discussions held during a CIRS multi-stakeholder workshop in December 2020 entitled ‘Reimagining medicine regulatory models: implementing fit-for-purpose sustainable activities for [...]
CIRS RD Briefing 79 – Use of advisory committees in Colombia
This Briefing provides an overview of how advisory committees can be used to support the regulatory decision-making process and considering the context of Latin American regulatory systems, aims to [...]
CIRS RD Briefing 78 – HTA outcomes in Australia, Canada and Europe 2015-2019
This Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory [...]
CIRS RD Briefing 77 – New drug approvals in six major authorities
This Briefing presents the results from the CIRS annual analysis of New Active Substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food [...]
CIRS RD Briefing 76 – Mexican therapeutic landscape
An efficient regulatory process can be reflected in measurable positive health impacts; conversely, activities that slow or impede regulatory efficiency and predictability can be detrimental. Recent developments in the [...]
CIRS RD Briefing 75 – Emergency Use Pathways (EUPs)
It has become clear that agencies have a number of pathways that can be used during public health emergencies for the authorisation of therapeutics and vaccines. Some of these [...]
CIRS RD Briefing 74 – OpERA programme
CIRS has collected regulatory assessment data for over 20 years, initially with ICH and ICH-observing countries. The OpERA programme, “Optimising Efficiencies in Regulatory Agencies (OpERA)”, was initiated through CIRS [...]
CIRS RD Briefing 73 – HTA outcomes 2014-18
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure that patient access to medicines of therapeutic value is not delayed. As part of an [...]
CIRS RD Briefing 71 – Trends in the regulatory landscape Latin America
To address the complex challenges in the global regulatory environment and the growing demand for patient access to new medicines, regulatory agencies in Latin America are actively engaging in regulatorystrengthening [...]
CIRS RD Briefing 72 – Trends in the regulatory landscape Asia
To address the complex challenges in the global regulatory environment and the growing demand for patient access to new medicines, regulatory agencies in Asia are actively engaging in regulatory-strengthening and [...]
CIRS RD Briefing 70 – New approvals in six regulatory authorities 2009-18
Major improvements in the regulatory environment as well as changes in strategies of multinational companies have led to a general decrease in the time to marketing authorisation and improved consistency [...]
CIRS RD Briefing 69: Review of HTA outcomes and timelines 2014-2017
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure that patient access to medicines of therapeutic value is not delayed. As part of [...]
CIRS RD Briefing 68: Regulatory and HTA decision making and access to medicines – the consequences of sequence
Historically, every jurisdiction with some form of regulatory agency capacity has undertaken the review of medicines as a first step in the market access process. This step is intended [...]
CIRS RD Briefing 67 – New approvals in six regulatory authorities 2008-2017
Major improvements in the regulatory environment as well as changes in strategies of multinational companies have led to a decrease in the time to marketing authorisation as well as [...]
CIRS RD Briefing 66 – Benefit-Risk Assessment Tool (BRAT)
A structured approach to benefit-risk assessment is required as the cornerstone of a consistent way to evaluate and communicate observations regarding a medicine’s benefit-risk profile. The Benefit Risk Action Team [...]
CIRS RD Briefing 65 – New approvals in six regulatory authorities 2007-2016
Over the last decade, 2007-2016, convergence in approval times as well as changes in strategies of multinational pharmaceutical companies have resulted in more new active substances (NASs) being internationalised, [...]
CIRS RD Briefing 64: Review of HTA outcomes and timelines 2014-2015
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure patient access to medicines of therapeutic value. As part of an ongoing study to [...]
CIRS RD Briefing 63: HTA process maps
This R&D Briefing summarises the background and methodology of process mapping with examples to demonstrate the steps involved in regulatory, HTA and coverage processes for new medicines. Process maps [...]
CIRS RD Briefing 62 – New drug approvals in ICH countries 2007-2016
There have been major improvements in the regulatory environment in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) countries over the [...]
CIRS RD Briefing 61: Building quality into decision-making processes
In 2015, CIRS initiated a programme in Quality Decision Making with the following aims: Evaluate the current decision-frameworks and understand the characteristics of different decision-making processes Assess the quality [...]
CIRS RD Briefing 60 – Early scientific advice from HTA agencies
This R&D Briefing 60 summarises highlights from the Technical Forum convened by CIRS on 11 December 2015, Heathrow, UK. Forum objectives: Identify companies’ current approaches to seeking early scientific [...]
CIRS RD Briefing 59 – New approvals in six regulatory authorities 2006-2015
The last decade, 2006-2015, has seen a continuation of the convergence and general decrease in the approval times amongst six major regulatory authorities, namely the European Medicines Agency (EMA), [...]
CIRS RD Briefing 58: Changing regulatory environment in Latin America
The aim of this Briefing is to review and summarise the findings from the major studies and interactions carried out by CIRS in LATAM in the last decade in [...]
CIRS RD Briefing 57 – New drug approvals in ICH countries 2005-2014
There have been major improvements in the regulatory environment in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) countries over the [...]
CIRS RD Briefing 56: Understanding the dynamics of China’s regulatory environment
Changes have occurred in the organisation and procedural activities of the China Food and Drug Administration (CFDA) and the Centre for Drug Evaluation (CDE). Initiatives that were designed to [...]
CIRS RD Briefing 55 – Approvals across six major authorities 2004-2013
As part of the ongoing study to monitor regulatory performance, CIRS has analysed the trends in new medicines’ approval between 2004 and 2013 by six regulatory authorities including Health [...]
CIRS RD Briefing 54 – Approvals in ICH countries 2004-2013
In 2013, the overall number of New Active Substances (NASs) approved by EMA, FDA and PMDA was comparable across the three agencies. Nevertheless, despite this similarity, the number of [...]
CIRS RD Briefing 53 – Factors influencing drug roll out to six mature markets
Objective: To review NASs first launched between 2005‐2010 and to determine their regulatory status as of 31 December 2012 in USA, Europe, Japan, Canada, Switzerland and Australia to identify [...]
CIRS RD Briefing 52 – New drug approvals in ICH countries 2003-2012
Active Substances (NASs) approved by both the FDA and PMDA represented the largest number of new medicines approved this decade. Regulatory approvals by EMA were lower than the other [...]
CIRS RD Briefing 51 – Submission lag time in the emerging markets
Lag Time (the time from which a product has been granted market authorisation in its first market to the time that its application is submitted for review by an [...]
CIRS RD Briefing 51 – New drug approvals in ICH countries 2002-2011
In 2011, the number of New Active Substances (NASs) approved in all the ICH countries increased compared to 2010, and FDA represented the largest number of new medicines approved [...]
CIRS RD Briefing 46: Building quality into regulatory activities
The Institute for Regulatory Science is currently involved in several activities related to quality as it applies to regulatory submissions and procedures, rather than the more conventional association with [...]
CIRS RD Briefing 50 – Cross-regional comparison of regulatory environment in emerging markets
The emerging markets of Asia-Pacific, the Middle East, Africa and Latin America are becoming increasingly important to pharmaceutical companies in their global strategies for the registration of new medicines [...]
CIRS RD Briefing 49 – Accessing the regulatory environment – Latin America
The Regulatory Agencies in Latin America share a common goal with the research-based pharmaceutical companies that they regulate. This is to ensure that new medicines become available to patients [...]
CIRS RD Briefing 48 – Assessing the regulatory environment – Middle East and Africa
The regulatory agencies in the Middle East and African region that were included in this study share a common goal with the research-based pharmaceutical companies that they regulate. This [...]
CIRS RD Briefing 47 – Accessing the regulatory environment – SE Asia and Western Pacific
The regulatory agencies in South East Asia and the Western Pacific share a common goal with the research-based pharmaceutical companies that they regulate. This is to ensure that new [...]
CIRS RD Briefing 44: Global drug development and regulatory review
Although it is the goal of most major pharmaceutical companies to achieve global development for new medicines with simultaneous submission of the regulatory dossier in the three main ICH [...]
CIRS RD Briefing 43: Current strategies in global drug development
A summary of the outcome of a survey carried out by the CMR International Institute for Regulatory Science among pharmaceutical companies. Although the pharmaceutical industry is moving towards integrated [...]
CIRS RD Briefing 45: Regulatory timelines in Saudi Arabia
This unique study is the first in-depth analysis of the trends and changes in the regulatory approval times for medicines in the Kingdom of Saudi Arabia. Not only have [...]
CIRS RD Briefing 42: Changing regulatory environment
A summary of the outcome of a survey carried out by the CMR International Institute for Regulatory Science among pharmaceutical companies and regulatory agencies. There was consensus that the [...]
CIRS RD Briefing 41 – Workshop on Regulatory Performance
Highlights from the workshop entitled 'Regulatory Performance: Critical Success Factors in Today’s Environment', held by the CMR International Institute for Regulatory Science in Washington D.C., 15-16 September 2003 In [...]
CIRS RD Briefing 40 – Pharmacogenetics and pharmacogenomics in drug development
Summarised results of a survey carried out among pharmaceutical companies and regulatory agencies, by the CMR International Institute for Regulatory Science Pharmacogenetic (PGt) and pharmacogenomic (PGx) technologies are being [...]
CIRS RD Briefing 39 – Regulating personalised medicine
Highlights of the workshop "Regulating personalised medicine" organised by the CMR International Institute for Regulatory Science, Nutfield Priory, Surrey, UK, 14-15 April 2003 The unravelling of the human genome [...]
CIRS RD Briefing 38 – Risk management
Highlights of the CMR International institute Workshop on Risk Management: The role of regulatory strategies in the development of new medicines, Washington DC, 12-13 December 2002 Following a number [...]
CIRS RD Briefing 37 – Adoption of ICH E5 in Asia Pacific
In 2001, CMR International conducted a study among pharmaceutical companies to evaluate their experience with regulatory authorities in Asia Pacific regarding the acceptance of foreign clinical data and adoption [...]
CIRS RD Briefing 36 – Impact of ICH E5 guideline
The ICH E5 Guideline was introduced in February 1998 and subsequently implemented by the regulatory authorities of the USA, EU and Japan. The purpose of this guideline is to [...]
CIRS RD Briefing 31 – Regulatory review times
CMR International R&D Briefing 31 - Profile of performance: Review times - is there still room for improvement? Key messages: Performance improvement initiatives established by regulatory authorities in the [...]
Ensuring efficiency and effectiveness of Joint Clinical Assessment (JCA) – Wang 2025
Background: This study explored the readiness and strategic considerations of companies and key stakeholders for the implementation of the Joint Clinical Assessment (JCA) under the European Health Technology Assessment [...]
Economic impact of reliance on an African regulator – Danks 2025
Background and Objectives The inherited backlog of 16,000 medicines applications of the South African Health Products Regulatory Authority (SAHPRA) was cleared through facilitated review pathways that included reliance on [...]
Suggested Improvements to the EAC-MRH Joint Review Process – Ngum 2025
Background In 2012, the East African Community Medicines Regulatory Harmonization (EAC-MRH) initiative was established to improve access to safe, effective, and high-quality medical products to patients in the East [...]
Comparison of good review practices in ECOWAS – Owusu-Asante 2025
Introduction: When implemented by national and regional regulatory agencies good review practices (GRevPs) support the timely high-quality review of medicines for enhanced patients’ availability to safe, quality and efficacious [...]
Evaluation of Zambian Medicines Regulatory Authority review process – Chisha 2024
Purpose: This study aimed to assess the current regulatory review process of the Zambia Medicines Regulatory Authority (ZAMRA) by identifying the key milestones and target timelines achieved for products [...]
Ngum 2024 – Evaluation of review models and timelines in the EAC-MRH
Introduction: Medicines regulatory harmonisation has been embraced by many national regulatory authorities (NRAs) to improve public health through faster availability of safe, high-quality, and effective medical products to patients [...]
Ngum 2024 – Evaluation of good review practices in the EAC-MRH
Introduction: The East African Community Medicines Regulatory Harmonisation (EAC-MRH) programme was established to address challenges faced by national regulatory authorities (NRAs) of the region. Work sharing through joint assessments [...]
Comparison of Three Regional Medicines Regulatory Harmonisation Initiatives in Africa
Background The African Medicines Regulatory Harmonisation (AMRH) Initiative was formed in 2009 and subsequently, three regional initiatives (East African Community Medicines Regulatory Harmonisation [MRH], Southern African Development Community [SADC]/ZaZiBoNa [...]
Evaluation of the Swissmedic regulatory framework for new active substances
Background: Swissmedic is a major regulatory agency that has been benchmarking its timelines for 20 years. To better understand the Swissmedic review times and to examine whether measures introduced to [...]
Impact of reliance on the regulatory performance of the South African Health Products Regulatory Authority
Introduction: The World Health Organization (WHO) advocates the use of reliance practices to enable national regulatory authorities (NRAs) to improve patients’ access to medicines. This study considered whether reliance review [...]
A comparison of regulatory decision patterns for oncology products to all other non-oncology products among Swissmedic, EMA and FDA
Consensus of regulatory decisions on the same Marketing Authorization Application (MAA) are critical for stakeholders. In this context, regulatory decision patterns from the Swissmedic (SMC), the US Food and [...]
Evaluation of Risk‑Based Approaches to the Registration of Medicines: Current Status Among African Regulatory Authorities
Background: Despite the worldwide need for increased access to safe and effective medicines, there is a lack of innovative medicines in many low- to middle-income countries. On the African [...]
Regulatory performance of the East African Community joint assessment procedure: The way forward for regulatory systems strengthening
Background: Seven national medicines regulatory authorities in the East African Community (EAC) have embraced regulatory reliance, harmonization and work sharing through the EAC Medicines Regulatory Harmonization programme. Measuring the [...]
Assessment of the effectiveness and efficiency of the West Africa medicines regulatory harmonization initiative by the pharma industry
Background: Following the establishment of Economic Community of West African States Medicines Regulatory Harmonization (ECOWAS-MRH) initiative in 2017, it was considered timely to carry out an evaluation of the current [...]
Regulatory, HTA and company interactions: the current landscape and future ecosystem for drug development, review and reimbursement
Background: Multi-stakeholder interactions have evolved at product and policy levels. There is a need to assess the current and future landscape of interactions between companies, and regulatory and HTA agencies [...]
Assessment of the effectiveness and efficiency of the West Africa medicines regulatory harmonization initiative by the member countries
Background: The West Africa Health Organization launched the West Africa Medicines Regulatory Harmonization Project (WA-MRH) in 2017 with the overarching objective to improve the availability of high-quality, safe and [...]
Evaluation of the Food and Drugs Authority, Ghana Regulatory Review Process: Challenges and Opportunities
Purpose: This study aimed to assess the current regulatory review process of the food and drugs authority (FDA) Ghana by identifying key milestones, target timelines, good review practices and [...]
Evaluation of the East African Community joint assessment procedure by pharmaceutical companies
Background: A 2021 study to determine the viewpoints among the seven member countries regarding the effectiveness (i.e., achieving the intended outcomes) and efficiency (i.e., achieving the intended outcomes in [...]
Evaluation of the East African Community joint assessment
Background: For almost a decade, the East African Community has implemented the Medicines Regulatory Harmonization (EAC-MRH) programme among its member states to harmonise technical requirements and standards for medical [...]
Building HTA/Payer Perspectives Into Drug Development
Background: The target product profile (TPP) outlines the desired profile of a target product aimed at a particular disease and is used by companies to plan clinical development. Considering the [...]
Evaluation of the performance of the Gulf centralised registration procedure
Background: The Gulf Centralised Committee for Drug Registration (GCC-DR), as part of the Gulf Health Council (GHC), enables the consolidated registration of pharmaceutical products throughout the member states of [...]
Industry evaluation of the efficiency and effectiveness of ZaZiBoNa
Introduction: The common technical document (CTD) format harmonised the requirements for the registration of medicines, which had traditionally differed from country to country, making it possible for countries to [...]
Regulatory evaluation of the efficiency and effectiveness of ZaZiBoNa
Introduction: ZaZiBoNa, the work-sharing initiative in the Southern African Development Community (SADC) that has been in operation for 8 years has successfully assessed over 300 dossiers/applications, with an overall [...]
Comparison of the registration process of Zimbabwe with Australia, Canada, Singapore and Switzerland
Background: Benchmarking regulatory systems of low- and middle-income countries with mature systems provides an opportunity to identify gaps, enhance review quality, and reduce registration timelines, thereby improving patients’ access [...]
Seeking early scientific advice from HTA agencies
There is a growing trend for pharmaceutical companies to seek scientific advice on drug development from a Health Technology Assessment (HTA) perspective, to improve the efficiency of their studies, [...]
Regulatory-HTA decision-making interface: what the medical writer should know
For a new medicine to reach patients, it must achieve both regulatory marketing authorisation and reimbursement from the payer. Because regulators assess the benefits and risks of a medicine [...]
Review models and timelines in the Southern African Development Community
Introduction: Regulatory reliance, harmonization and work sharing have grown over the last few years, resulting in greater sharing of work and information among regulators, enabling efficient use of limited [...]
Good Review Practices in the Southern African Development Community
Introduction: National medicines regulatory agencies are faced with challenges including limited resources and technical capacity, resulting in countries collaborating and sharing resources to improve efficiency of the review process to [...]
Keyter et al 2021 – Impact of reliance on South African review
Background: The aims of this study were to compare the overall regulatory review timelines achieved by the South African Health Products Regulatory Authority (SAHPRA) in 2020 to the timelines historically [...]
Bujar et al 2021 – Transparency in EMA and FDA decision making
Although it cannot be expected that different medicines’ regulatory agencies always reach the same review outcome, it is important that decision making is documented and communicated to ensure transparency. [...]
Bujar et al 2021 – Value of Facilitated Regulatory Pathways
Background: Despite the growing application of facilitated regulatory pathways (FRPs), little attention has focused on assessing the perception of pharmaceutical companies regarding their usefulness beyond increasing timeliness. Objectives: The [...]
Sithole et al 2021 – Regulatory review process in Zimbabwe
Purpose: The aims of this study were to assess the current regulatory review process of the Medicines Control Authority of Zimbabwe (MCAZ), identify key milestones and target timelines, evaluate [...]
Wang et al 2020 – Companies’ HTA strategies and practices
Background: Health technology assessment (HTA) has increased in importance in supporting payer decision making by assessing the relative effectiveness and cost effectiveness of new medicines. Thus, pharmaceutical companies need to [...]
Keyter et al 2020 – A proposed regulatory review model to support SAHPRA
Background: National regulatory agencies of various sizes and maturity levels, including the South African Health Products Regulatory Authority (SAHPRA), have had to revise systems and re-engineer processes in order [...]
Liberti et al 2020 – Evaluation of the Caribbean Regulatory System centralised assessment process
Background: The Caribbean Regulatory System is a centralized medicine assessment procedure established to serve the needs of the Member States of the CARICOM region. In order to better understand [...]
Wang et al 2020 – Benchmarking HTA agencies: methodological challenges and recommendations
Objectives: The objectives of the study were to establish a benchmarking tool to collect metrics to enable increased clarity regarding the differences and similarities across health technology assessment (HTA) [...]
An independent perspective on the East African Community Medicines Regulatory Harmonisation initiative
African regulators are taking bold transformative steps to optimise the effective and efficient use of their agency resources to assure access to quality, safe and effective medicines. In particular, [...]
Bujar et al 2020 – A Process for Evaluating Quality Decision-Making Practices
Background: The development of a medicine is not only underpinned by good science but also by Quality Decision-Making Practices (QDMPs). Indeed, it is important to ensure that all organisations [...]
Rodier et al 2020 – Use of the CPP in 18 maturing markets
Background: The certificate of pharmaceutical product (CPP) was implemented to accelerate the availability of new drugs in developing countries by providing evidence of the quality of products and reducing [...]
Keyter et al 2020 – Can standardisation of the Public Assessment Report improve benefit-risk communication?
Background: National regulatory authorities (NRAs) make the decision to register a medicine based on an assessment of its benefits and risks and publicly available assessment reports are used as [...]
Bujar et al 2020 – Documentation of Quality Decision-Making Practices
Background: Pharmaceutical companies and regulatory agencies endeavor to relate their decision making with outcomes to improve future decision making and to ensure that gained knowledge is fed back into [...]
Patel et al 2020 – Analysis of regulatory review timelines for ANVISA
Background: The Brazilian health regulatory agency (Agência Nacional de Vigilância Sanitária, ANVISA) has embarked on transformational initiatives to fulfill its mandate to provide timely access to safe, effective, and [...]
Mohd Sani et al 2020 – Evaluation of the Malaysian regulatory process using OpERA methodology
Introduction: The National Pharmaceutical Regulatory Agency (NPRA) embarked on a regulatory-strengthening program and is evaluating its processes. Optimising Efficiencies in Regulatory Agencies (OpERA) is a regulatory-strengthening program that provides [...]
Sithole et al 2020 – Evaluating the success of ZaZiBoNa
The Southern African Development Community (SADC) collaborative medicines registration initiative ZaZiBoNa is a successful regional work-sharing initiative on the African continent. This paper reviews the history of the ZaZiBoNa [...]
Keyter et al 2020 – Implementation of a Framework for an Abridged Review Using Good Reliance Practices: Optimising the Medicine Regulatory Review Process in South Africa
Background This study sought to identify criteria and current practices for implementing an abridged review process and understanding barriers and enablers in utilizing reliance models and to offer recommendations [...]
Mashaki Ceyhan et al 2020 – Patients’ perspectives of the pharmaceutical regulatory and reimbursement system in Istanbul, Turkey
The aim of this study was to explore patients’ knowledge and perspectives in Istanbul, Turkey about the pharmaceutical regulatory review and reimbursement processes with respect to access to new [...]
Koyuncu et al 2020 – Evaluation of the performance of the Turkish regulatory agency: recommendations for improved patients’ access to medicines
Background: This study was to evaluate the Turkish regulatory review process and timelines between 2016 and 2018 with a view to assess the changes that had taken place since [...]
Keyter et al 2019 – Evaluation of the performance of the South African regulatory agency
Background: Timely access to new medicines may be addressed through strengthening of registration efciencies and timelines by establishing and refning value-added registration processes, resources, and systems. The aims of [...]
Kühler et al 2019 – To what degree are review outcomes aligned for new active substances between the EMA and the US FDA?
Objective To compare review outcome alignment between European Medicines Agency (EMA) and US Food and Drug Administration (FDA) for medicines approved by both agencies in the time period 2014–2016. Design [...]
McAuslane et al 2019 – Confluence of Accelerated Regulatory and HTA Access Pathways
There is a growing interest in aligning accelerated regulatory pathways with flexible access and reimbursement pathways to expedite the equitable availability of high-quality, safe, and effective medicines that provide [...]
Keyter et al 2019 – South African Medicines Control Council: Comparison of Its Registration Process
Introduction: Comparisons between regulatory authorities of similar size and regulatory characteristics facilitate value-added benchmarking and provide insight into regulatory performance. Such comparisons highlight areas for improvement as authorities move [...]
Bujar et al 2019 – Quality decision making in Health Technology Assessment
Background: To evaluate the quality of the decision-making processes of pharmaceutical companies during medicines development for evidence generation to support reimbursement of new medicines and the appraisal recommendation decision-making process [...]
Bujar et al 2019 – Reliability and relevance of a decision-making instrument
Introduction: The Quality of Decision-Making Orientation Scheme (QoDoS) was developed to provide organisations involved in submission, approval and reimbursement of new medicines with a tool to improve the quality [...]
Wang et al 2018 – Building Synergy between Regulatory and HTA Agencies
Objectives: To evaluate the current practice of companies and agencies to assess the changes made in aligning regulatory and health technology assessment (HTA) stakeholders; to identify areas of commonality [...]
Haqaish et al 2017 – Jordan Food and Drug Administration: Comparison of its Registration Process with Australia, Canada, Saudi Arabia and Singapore
Objective: This study compares the current regulatory review process and good review practices at the Saudi Food and Drug Authority (SFDA) with those of regulatory agencies in Australia, Canada, [...]
Hashan et al 2016 – Saudi Arabia FDA: An Evaluation of the Registration Process and Good Review Practices in Saudi Arabia in Comparison with Australia, Canada and Singapore
Objective: This study compares the current regulatory review process and good review practices at the Saudi Food and Drug Authority (SFDA) with those of regulatory agencies in Australia, Canada, [...]
Al-Rubaie et al 2015 – Agency and industry views of the efficiency of the Gulf centralised registration procedure
The aim of this study was to examine the views and experiences of the Gulf Cooperation Council (GCC) states and pharmaceutical companies to identify the strengths and weaknesses of [...]
Walker et al 2015 – Universal Framework for the Benefit-Risk Assessment of Medicines
A universal framework for the evaluation of the benefit-risk assessment of medicines during development by pharmaceutical companies and in the regulatory review by regulatory authorities is considered of value, [...]
Al-Rubaie et al 2014 – Evaluation of the Gulf centralised registration procedure
The aim of the study was to evaluate the Gulf Cooperation Council (GCC) centralized regulatory review process. Regulatory review times—including submission and application dates for new active substances (NASs) [...]
Liu et al 2013 – Characterising Good Review Practices across APEC agencies
As a first step in the implementation of the Asia-Pacific Economic Cooperation (APEC) Best Regulatory Practice Project, the Centre for Innovation in Regulatory Science conducted a gap analysis survey [...]
Allen et al 2013 – Archetypes for non-ranking classification and comparison of European HTA systems
Introduction: European countries are increasingly utilising health technology assessment (HTA) to inform reimbursement decision-making. However, the current European HTA environment is very diverse, and projects are already underway to [...]
Salek et al 2012 – Scorecards to Assess the Quality of a Regulatory Submission and Its Review
An efficient review depends not only on timely approval but also on ensuring the quality of the process from construction of the dossier to the ultimate regulatory decision. Two [...]
Hirako et al 2007 – Comparison of the drug review process at five international regulatory agencies
Regulatory approval time is a key metric that is used to evaluate the performance of regulatory agencies. A new methodology has been developed to compare the regulatory review process [...]
Regulatory Collaboration and System Strengthening – Workshop Synopsis
This CIRS multi-stakeholder workshop examined success factors for strengthening regulatory systems to support the implementation of collaborative models.
Workshop Report – Regulatory and HTA collaborative models
In this workshop, CIRS brought together senior representatives from regulators, HTA agencies, pharmaceutical companies, payers, academics and patient organisations to discuss the impact of regulatory and HTA collaborative models [...]
Review of 2024 CIRS activities
We're pleased to share a high-level summary of what CIRS got up to last year, including key research outputs and meetings. Our full 2024 Annual Report will be published [...]
Workshop Synopsis – Regulatory and HTA collaborative models
In this workshop, CIRS brought together senior representatives from regulators, HTA agencies, pharmaceutical companies, payers, academics and patient organisations to discuss the impact of regulatory and HTA collaborative models [...]
Workshop Report – Vaccine regulatory and funding approaches
Over the last four years there has been much greater attention paid by industry, regulators, health technology assessment (HTA) bodies and the general public to vaccines for a range [...]
Monitoring implementation and adherence to ICH guidelines
Background: This study was built on the previous 2019 and 2021 assessments where the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) selected CIRS [...]
Workshop Report – Ensuring efficient and effective implementation of joint clinical assessment (JCA)
The Regulation (EU) 2021/2282 on health technology assessment (HTAR) reflects a significant step towards harmonising the clinical assessment in HTA decision making across EU Member States. It aims to [...]
2024 Workshop Synopsis – Evolving regulatory and funding approaches for vaccines
In this workshop, CIRS brought together senior representatives from the international pharmaceutical industry, regulatory agencies, National Immunisation Technical Advisory Groups (NITAGs), HTA agencies, payers and academia to identify challenges [...]
2024 Workshop Synopsis – Ensuring efficient and effective implementation of joint clinical assessment (JCA)
In this workshop, CIRS brought together senior representatives from HTA agencies, pharmaceutical companies, payers and patient organisations to discuss their readiness for the EU HTA Regulation (HTAR) being applied [...]
2024 Workshop report – Reliance and regional review models
Faced with increasingly complex technologies and novel evidence generation techniques, regulatory agencies are being challenged to work in new ways. There is pressure on them to be agile and [...]
CIRS 2023 Annual Report
We're delighted to present our latest Annual Report, which provides a summary of the projects, publications, multi-stakeholder workshops and Technical Fora from Regulatory and HTA workstreams undertaken in 2023. [...]
2024 Workshop Synopsis – What is needed for risk-based approaches to work effectively and efficiently?
In this workshop, CIRS brought together senior representatives from regulatory agencies, pharmaceutical companies and academia from 18 countries across the Americas, Africa, Asia and Europe, to examine risk-based approaches [...]
2023 Workshop report – Review and reimbursement frameworks for rare disease products
There are an estimated 300 million people across the world affected by around 7000 known rare diseases. Challenges in bringing treatments to market for these conditions include small patient [...]
2023 Workshop report – Uncertainty in the development of new medicines
This CIRS workshop brought together companies and agencies (HTA and Regulatory) to discuss the sources of uncertainty that are being built in, by the way medicines development has evolved [...]
2023 Workshop Synopsis – Regulatory and reimbursement frameworks for rare disease products
This multi-stakeholder workshop consisted of a series of presentation sessions and three parallel breakout discussions. Presentations explored trends in regulatory and HTA approvals of orphan products [...]
2023 Workshop report – New ways of working for medicines development
CIRS brought agencies and companies together in a workshop to discuss new ways of working and how the regulatory and HTA landscape in mature and maturing countries should evolve [...]
2022 Workshop report – Building on regulatory and HTA agilities for high unmet need
In 2020, coronavirus disease (COVID-19) spread rapidly around the world and halted regular social, business, and research activities. The pandemic impeded normal functioning across several fields, and businesses and [...]
2022 Workshop report – Collaborative models for regionalisation, work and information sharing: How do these fit into the regulatory toolkit?
This workshop looked at how maturing markets are building risk-based approaches into regulatory assessment, building on recent CIRS workshops in Singapore (2019) and South Africa (2018). The workshop also [...]
CIRS 2022 Annual Report
We're delighted to present our latest Annual Report, which provides a summary of the projects, publications and Multi-stakeholder Workshops, Technical Fora, Industry Discussion Meetings, and Impact Case Studies from [...]
2022 Workshop report – How has the pandemic accelerated the acceptance and utility of RWD/RWE in regulatory/HTA decision making?
This workshop builds on the outcomes of the CIRS Professor Breckenridge memorial workshop in December 2020, as well as the 2021 workshop on utilisation of digital technologies in clinical [...]
2021 Workshop report – Regulatory and HTA landscape in Asia and Latin America
As regulatory systems mature with demand for new innovative treatments, jurisdictions are seeking to introduce more comprehensive healthcare systems; this is often accompanied by efforts to initiate health technology [...]
CIRS 2021 Annual Report
We're delighted to present our latest Annual Report, which provides a summary of CIRS projects, publications and workshops undertaken last year as well as case studies depicting CIRS’ impact [...]
2021 Workshop report – Digital technologies for clinical evidence generation
Digitisation and digital health technologies are transforming clinical development; companies, regulators and Health Technology Assessment (HTA) agencies are looking to derive actionable insights from the data being generated. This [...]
2021 Workshop report – Regulatory, HTA and payer interactions and collaborations
Over the last five years, regulatory and HTA interactions, as well multi-HTA and multi-regulatory interactions and collaborations, have evolved in thinking and mutual activities both at a product level [...]
2020 Workshop report – Reimagining regulatory models
As the regulatory landscape changes to meet new challenges, such as increasingly sophisticated medical innovations, fundamental questions are being raised: What is the role of a ‘modern’ regulator today? [...]
2021 Project report – Monitoring implementation and adherence to ICH guidelines
Background: This study was built on the previous 2018/2019 assessment where ICH selected the Centre for Innovation in Regulatory Science (CIRS) to collaborate on the development and the conduct [...]
2020 Workshop report – Effectiveness of the regulatory approval process
This workshop was part of a series of global development workshops that brought together mature and maturing regulatory agencies. These workshops successively built on one another and evolved from [...]
CIRS 2020 Annual Report
We're delighted to present our latest Annual Report, which provides a summary of CIRS projects and workshops undertaken in 2020 as well as case studies depicting CIRS’ impact on [...]
2019 Workshop report – Optimising the regulatory review process by evaluating performance and addressing good reliance practices
This workshop was part of a series of workshops on reliance, which continues to be an important area of focus for CIRS. It builds on the outcomes of a [...]
2019 Workshop report – Identifying and understanding uncertainty during development
This workshop was a follow-on from a collaborative forum held in Utrecht in 2018 entitled What new research can enable a joint approach by regulatory and HTA agencies to [...]
CIRS 2019 Annual Report
We're delighted to present the inaugural CIRS Annual Report, which provides a summary of projects and workshops undertaken in 2019, as well as a historical perspective of CIRS achievements [...]
Project report – Monitoring implementation and adherence to ICH guidelines
Background: According to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Articles of Association, the regulatory members of ICH are expected to implement [...]
Executive Colloquium report – What is the value and return on investment for our company to maintain a regulatory policy function?
In June 2019, the Centre for Innovation in Regulatory Science (CIRS) held an Executive Colloquium in Rockville, MD, USA that brought together representatives from multinational pharmaceutical companies to gauge [...]
2018 Forum report – Managing uncertainties for products using early access pathways
In November 2018, a collaborative forum presented by CIRS and the Utrecht University WHO Collaborating Centre for Pharmaceutical Policy and Regulation brought together regulatory, HTA, industry and academic perspectives [...]
2017 Workshop report – Flexible regulatory/access pathways
The aim of this workshop was to bring together companies, patient representatives and HTA, payer and regulatory agencies to discuss current perspectives and opportunities for Facilitated Regulatory Pathways (FRPs) [...]
2018 Workshop report – Practical implementation of reliance models
This Workshop was a follow-on from that held in 2017 in Sao Paulo entitled “Facilitating the review of new medicines through risk-based evaluations: How can a stratification process be [...]
2017 Workshop report – Facilitating review through risk-based evaluations
This Workshop built on previous CIRS global development workshops as well the work being undertaken by various groups in the areas of good regulatory and review practices and focused [...]
2016 Workshop report – Commonality in evidentiary requirements
Commonality in evidentiary requirements across regulatory and HTA stakeholders 21-22 SEPTEMBER 2016, SURREY, UK Workshop objectives Discuss the progress made to align evidentiary requirements, what the drivers have been [...]
2016 Workshop report – RWD to RWE for assessing efficacy and effectiveness
Real-world data to real-world evidence for assessing efficacy and effectiveness: Opportunities and challenges for new medicines development, regulatory review and health technology assessment 23-24 JUNE 2016 TYSONS CORNER, VIRGINIA, [...]
2016 Workshop report – Key performance metrics for regulatory processes
WHAT ARE THE KEY PERFORMANCE METRICS THAT AGENCIES AND COMPANIES SHOULD USE TO MEASURE REGULATORY PROCESSES AND PRACTICES TO FACILITATE THE LICENSING OF NEW MEDICINES? 3-4 FEBRUARY 2016, KUALA [...]
2015 Workshop report – Patient involvement in review and reimbursement
What is the patient’s role in informing the decision process for approval and reimbursement of new medicines? 7-th October 2015, Windsor, UK This workshop built on CIRS workshops on [...]
2013 Workshop report – Commonality across decision frameworks used by HTA and regulatory agencies
IS THERE A COMMONALITY ACROSS THE STRUCTURED DECISION FRAMEWORKS USED BY HTA AND REGULATORY AGENCIES? 1st-2nd October 2013, Surrey, UK This Workshop was designed to bring together the various [...]
Role of Regional Initiatives in the Operationalisation of the African Medicines Agency: Contribution of the EAC-MRH Initiative
“This book teaches us important lessons that we will need to consider in our collaboration work with the African Medicines Agency. I am confident that this research will be [...]
Evolution of HTA practice and approaches from the perspectives of HTA agencies and pharmaceutical industry
Health technology assessment (HTA) has emerged as an important tool to support healthcare decision-makers to make rational reimbursement decisions, with the ultimate purpose of promoting an efficient healthcare system. [...]
Regulatory work-sharing initiative in Africa: ZaZiBoNa, past, present and future
PREFACE: The role of regulatory authorities in the health system is to ensure the quality, safety and efficacy of medical products. It is acknowledged that regulatory authorities are at [...]
Roadmap for Regulatory Performance
Every regulatory authority in the world has an ambition to improve its performance. In order to achieve this, agencies often establish performance indicators which they use for measuring and [...]
Quality decision-making practices
This book compiles the presentations and syndicate discussions from a CIRS workshop that brought together representatives from international pharmaceutical companiesand regulatory authorities from Europe, US, Canada and Australia and [...]
Globally Applicable Facilitated Regulatory Pathways to Improve Equitable Access to Medicines
A variety of approaches have been developed to accelerate the regulatory review of medicines. We characterise these various expedited pathways as facilitated regulatory pathways (FRPs): regulatory pathways designed to accelerate [...]
Frequency and Variation of Clock-Stop During EMA Assessment for Oncology Products – Implication on JCA Timelines
Objectives: The implementation act adopted for the HTA Regulation (HTAR) defined the timelines of scoping, submission and assessment and output of Joint Clinical Assessment (JCA), which will be in [...]
Rare Disease Product Approvals: The Changing Regulatory And HTA Landscape Between 2018-2022
Background Globally, 7,000 rare diseases affecting 300 million people pose development challenges with small patient populations. Developing medicines for rare diseases requires innovation. Despite regulatory incentives, challenges for HTA [...]
Navigating HTA Requirements During Development Through Early HTA Scientific Advice
Background Pharmaceutical companies have been actively taking early scientific advice from health technology assessment (HTA) agencies during development, with the aim to understand the HTA evidentiary requirements. The evolving [...]
Assessing the use of risk-based approaches in four major agencies
Introduction Over the last years, several regulatory agencies have developed risk-based approaches for the regulatory assessment of marketing authorisations of New Active Substances (NASs) as strategies to efficiently use [...]
HTA Timelines and Outcomes for MHRA-Approved NASs via Reliance/Work-sharing Routes
During ISPOR Europe 2023 in Copenhagen, Belen Sola presented a poster entitled ‘Study of HTA Timelines and Outcomes for MHRA-Approved NASs in the Post-Brexit UK via Reliance/Work-sharing Routes'. Background: [...]
Measuring time to market for new medicines in 7 Asian countries between 2016-21, following review by US FDA or EMA
During the DIA Global Annual Meeting 2023, Adem Kermad, Magda Bujar and Neil McAuslane developed and presented a poster in which they shared the results, recommendations and conclusions about [...]
Evaluation of the Effectiveness and Efficiency of Ten Years’ Experience with the East Africa Community Joint Assessment
During the DIA Global Annual Meeting 2023, Nancy Yang-Ngum developed and presented a poster in which she shared the results, recommendations and conclusions about the topic "Evaluation of the [...]
Evaluation of the Regulatory Review Process of the FDA Ghana: Challenges and Opportunities for Improvement
During the DIA Global Annual Meeting 2023, Mercy Owusu-Asante developed and presented a poster in which she shared the results, recommendations and conclusions about the topic "Evaluation of the [...]
Evaluation of the impact of reliance on the regulatory performance in the South African Health Products Regulatory Authority
During the DIA Global Annual Meeting 2023, Lorraine Danks, Boitumelo Semete-Makokotlela, Sam Salek and Stuart Walker developed and presented a poster in which they shared the results, recommendations and [...]
A comparison of the Regional Medicines Regulatory Harmonisation Projects in East, West and Southern Africa.
During the DIA Global Annual Meeting 2023, Tariro Sithole, Nancy Ngum, Mercy Owusu-Asante, Stuart Walker and Sam Salek developed and presented a poster in which they shared the results, [...]
December 2019 Slide of the Month
December's Slide of the Month is taken from a CIRS poster recently presented at ISPOR Europe 2019. Does FDA Breakthrough Designation affect HTA recommendation in terms of timing and outcome? [...]