HTADock: benchmarking HTA agency performance
What is HTADock?
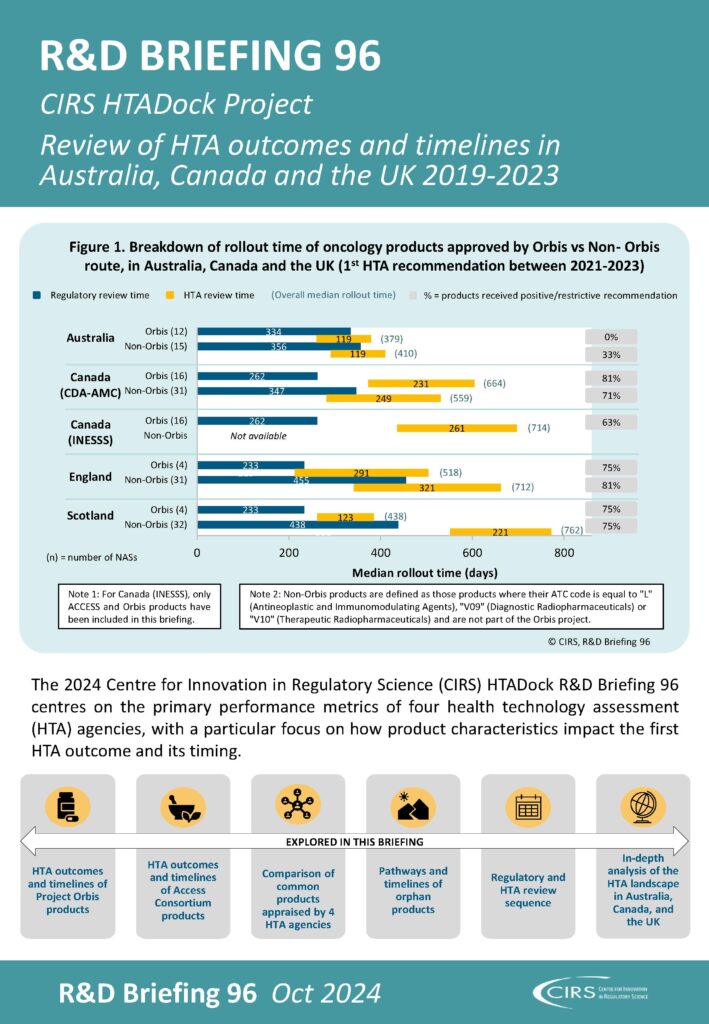
HTADock is an annual CIRS metrics study aiming to facilitate performance improvement in HTA agencies and increase transparency of the outcomes and timelines of HTA assessments. The HTADock database collects data from the public domain on new active substances (NASs) appraised by nine agencies (Australia, Canada, England, France, Germany, the Netherlands, Poland, Scotland and Sweden) since 2014. This includes information on first HTA recommendations, time from regulatory submission to first HTA recommendation, characteristics of products, regulatory pathways used and HTA implementation. The findings of the HTADock study are published annually in an R&D Briefing (see R&D Briefing 95 for the latest study).
How was it developed?
HTADock was derived from a collaborative project carried out in 2013/14 with ten HTA agencies, which identified common milestones in the HTA process and mapped jurisdiction-specific processes against the agreed metrics. The benchmarking methodology was published in International Journal of Technology Assessment in Health Care, to demonstrate the feasibility of benchmarking HTA performance metrics.
What has been the impact of HTADock?
Supporting discussions within HTA agencies: CIRS has utilised HTADock R&D Briefings over the years to stimulate interactions with HTA agencies, some of whom have then used these insights to support internal discussions on performance improvement. We also know of HTA agencies that have utilised HTADock data to demonstrate their assessment timelines with their Ministers of Health.
Informing on regulatory-HTA alignment: Regulatory agencies have expressed interest in HTADock data showing the synchronisation of regulatory and HTA decisions.
Further research on HTA trends: CIRS member companies have used the HTADock Briefings for their own research purposes, for example, to commission follow-up projects that help to inform company strategy. CIRS has also built upon HTADock findings to answer the following research questions:
- Does FDA Breakthrough Designation affect HTA recommendation in terms of timing and outcome?
- Do conditional regulatory pathways affect HTA recommendation?
- Do parallel regulatory-HTA reviews affect time to HTA decision?