During the DIA Global Annual Meeting 2023, Nancy Yang-Ngum developed and presented a poster in which she shared the results, recommendations and conclusions about the topic “Evaluation of the Effectiveness and Efficiency of Ten Years’ Experience with the East Africa Community Joint Assessment”
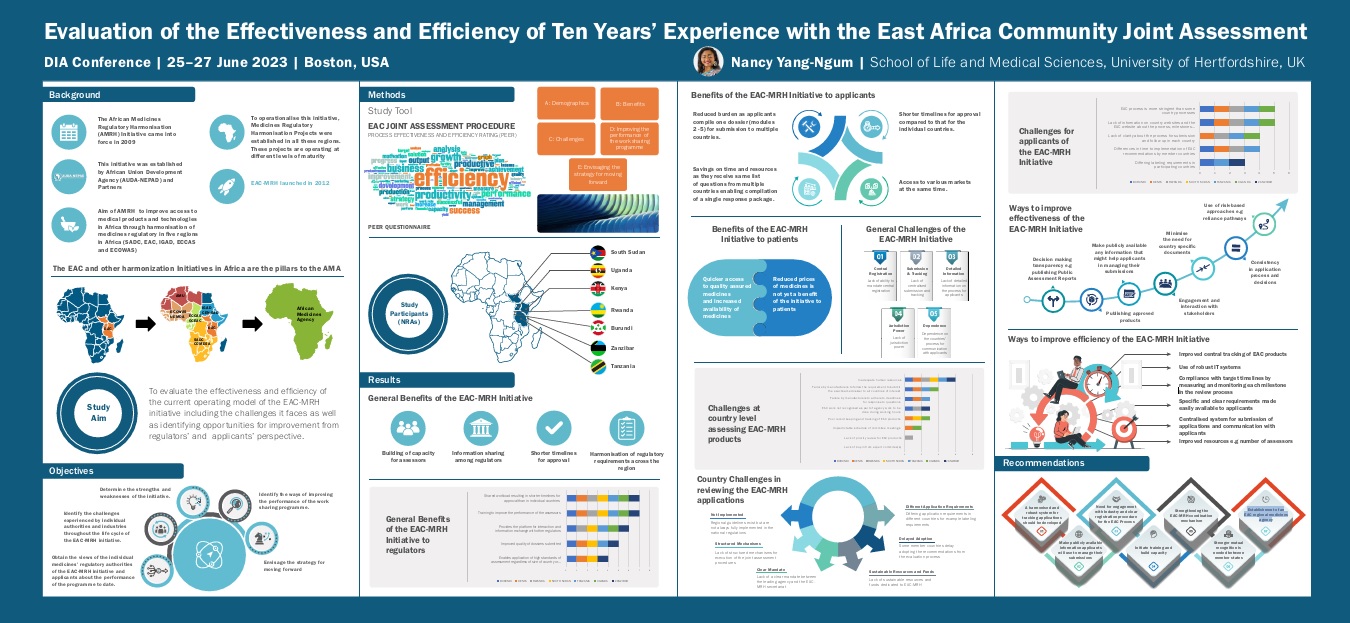
Background:
- The African Medicines Regulatory Harmonisation (AMRH) Initiative came into force in 2009.
- This initiative was established by African Union Development Agency (AUDA-NEPAD) and Partners.
- Aim of AMRH to improve access to medical products and technologies in Africa through harmonisation of medicines regulatory in five regions in Africa (SADC, EAC, IGAD, ECCAS and ECOWAS)
- To operationalise this initiative, Medicines Regulatory Harmonisation Projects were established in all these regions. These projects are operating at different levels of maturity.
- EAC-MRH launched in 2012.
Aim:
To evaluate the effectiveness and efficiency of the current operating model of the EAC-MRH initiative including the challenges it faces as well as identifying opportunities for improvement from regulators’ and applicants’ perspective.
Objectives:
- Obtain the views of the individual medicines’ regulatory authorities of the EAC-MRH initiative and applicants about the performance of the programme to date.
- Identify the challenges experienced by individual authorities and industries throughout the life cycle of the EAC-MRH initiative.
- Determine the strengths and weaknesses of the initiative.
- Identify the ways of improving the performance of the work sharing programme.
- Envisage the strategy for moving forward.
Methods: The Process Effectiveness and Efficiency Rating (PEER) questionnaire was answered by the EAC Joint Assessment Procedure participants: South Sudan, Uganda, Kenya, Rwanda, Burundi, Zanzibar and Tanzania. The PEER questionnaire is a study tool formed of five sections such as demographics, benefits, challenges, improving the performance of the work-sharing programme and envisaging the strategy for moving forward.
Recommendations:
- A harmonised and robust system for tracking applications should be developed.
- Make publicly available information applicants will use to manage their submissions.
- Need for engagement with Industry and clear registration procedure for the EAC Process.
- Initiate training and build capacity.
- Strengthening the EAC-MRH coordination mechanism.
- Stronger mutual recognition is needed between member states.
- Establishment of an EAC regional medicines agency.