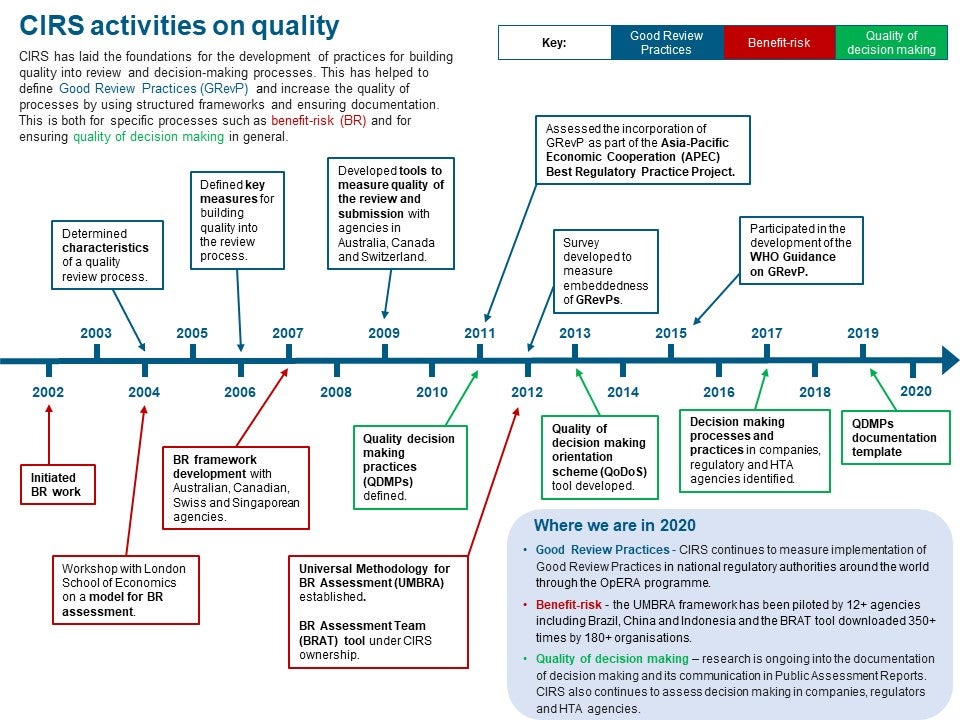
We’re delighted to present the inaugural CIRS Annual Report, which provides a summary of projects and workshops undertaken in 2019, as well as a historical perspective of CIRS achievements over the past decade or more (for example, see our quality timeline below).
CIRS has a rich history of helping to improve the regulatory and access landscape through its work in the areas of metrics, quality and alignment. As regulatory science continues to evolve, we will be looking to go further still including examining how agencies can improve their effectiveness.
“CIRS is a unique organisation. Every organisation makes such a claim, but the ability of CIRS to connect and actively involve government (regulators, HTA and payers), academia and industry to identify major research priorities in the clinical development, regulatory evaluation and funding of medicines is genuinely unique.”
– Adj Prof John Skerritt, Chair of the CIRS Scientific Advisory Council and Deputy Secretary of the Australian Government Department of Health.