During the DIA Global Annual Meeting 2023, Adem Kermad, Magda Bujar and Neil McAuslane developed and presented a poster in which they shared the results, recommendations and conclusions about the topic ” Measuring time to market for new medicines in 7 Asian countries between 2016-21, following review by US FDA or EMA”
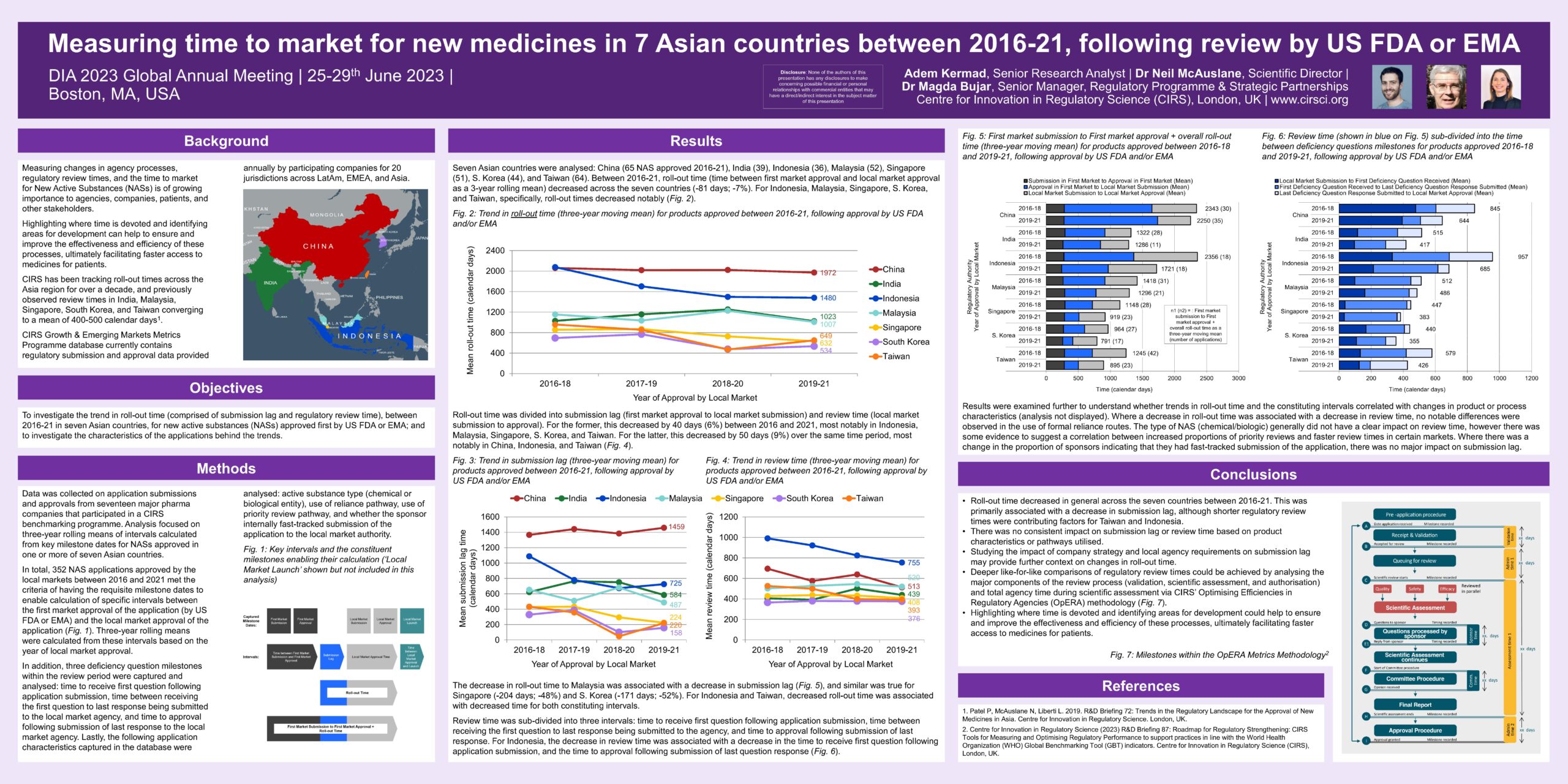
Background: Measuring changes in agency processes, regulatory review times, and the time to market for New Active Substances (NASs) is of growing importance to agencies, companies, patients, and other stakeholders.
Highlighting where time is devoted and identifying areas for development can help to ensure and improve the effectiveness and efficiency of these processes, ultimately facilitating faster access to medicines for patients.
CIRS has been tracking roll-out times across the Asia region for over a decade, and previously observed review times in India, Malaysia, Singapore, South Korea, and Taiwan converging to a mean of 400-500 calendar days.
CIRS Growth & Emerging Markets Metrics Programme database currently contains regulatory submission and approval data provided annually by participating companies for 20 jurisdictions across LatAm, EMEA, and Asia.
Objective: To investigate the trend in roll-out time (comprised of submission lag and regulatory review time) between 2016-21 in seven Asian countries, for new active substances (NASs) approved first by US FDA or EMA; and to investigate the characteristics of the applications behind the trends.
Methods: Data was collected on application submissions and approvals from seventeen major pharma companies that participated in a CIRS benchmarking programme. Analysis focused on three-year rolling means of intervals calculated from key milestone dates for NASs approved in one or more of seven Asian countries.
In total, 352 NAS applications approved by the local markets between 2016 and 2021 met the criteria of having the requisite milestone dates to enable calculation of specific intervals between the first market approval of the application (by US FDA or EMA) and the local market approval of the application (Fig. 1). Three-year rolling means were calculated from these intervals based on the year of local market approval.
In addition, three deficiency question milestones within the review period were captured and analysed: time to receive first question following application submission, time between receiving the first question to last response being submitted to the local market agency, and time to approval following submission of last response to the local market agency.
Lastly, the following application characteristics captured in the database were analysed: active substance type (chemical or biological entity), use of reliance pathway, use of priority review pathway, and whether the sponsor internally fast-tracked submission of the application to the local market authority.
Conclusions:
Roll-out time decreased in general across the seven countries between 2016-21. This was primarily associated with a decrease in submission lag, although shorter regulatory review times were contributing factors for Taiwan and Indonesia.
There was no consistent impact on submission lag or review time based on product characteristics or pathways utilised.
Studying the impact of company strategy and local agency requirements on submission lag may provide further context on changes in roll-out time.
Deeper like-for-like comparisons of regulatory review times could be achieved by analysing the major components of the review process (validation, scientific assessment, and authorisation) and total agency time during scientific assessment via CIRS’ Optimising Efficiencies in Regulatory Agencies (OpERA) methodology (Fig. 7).
Highlighting where time is devoted and identifying areas for development could help to ensure and improve the effectiveness and efficiency of these processes, ultimately facilitating faster access to medicines for patients.