During the DIA Global Annual Meeting 2023, Lorraine Danks, Boitumelo Semete-Makokotlela, Sam Salek and Stuart Walker developed and presented a poster in which they shared the results, recommendations and conclusions about the topic “Evaluation of the impact of reliance on the regulatory performance in the South African Health Products Regulatory Authority”
Background:
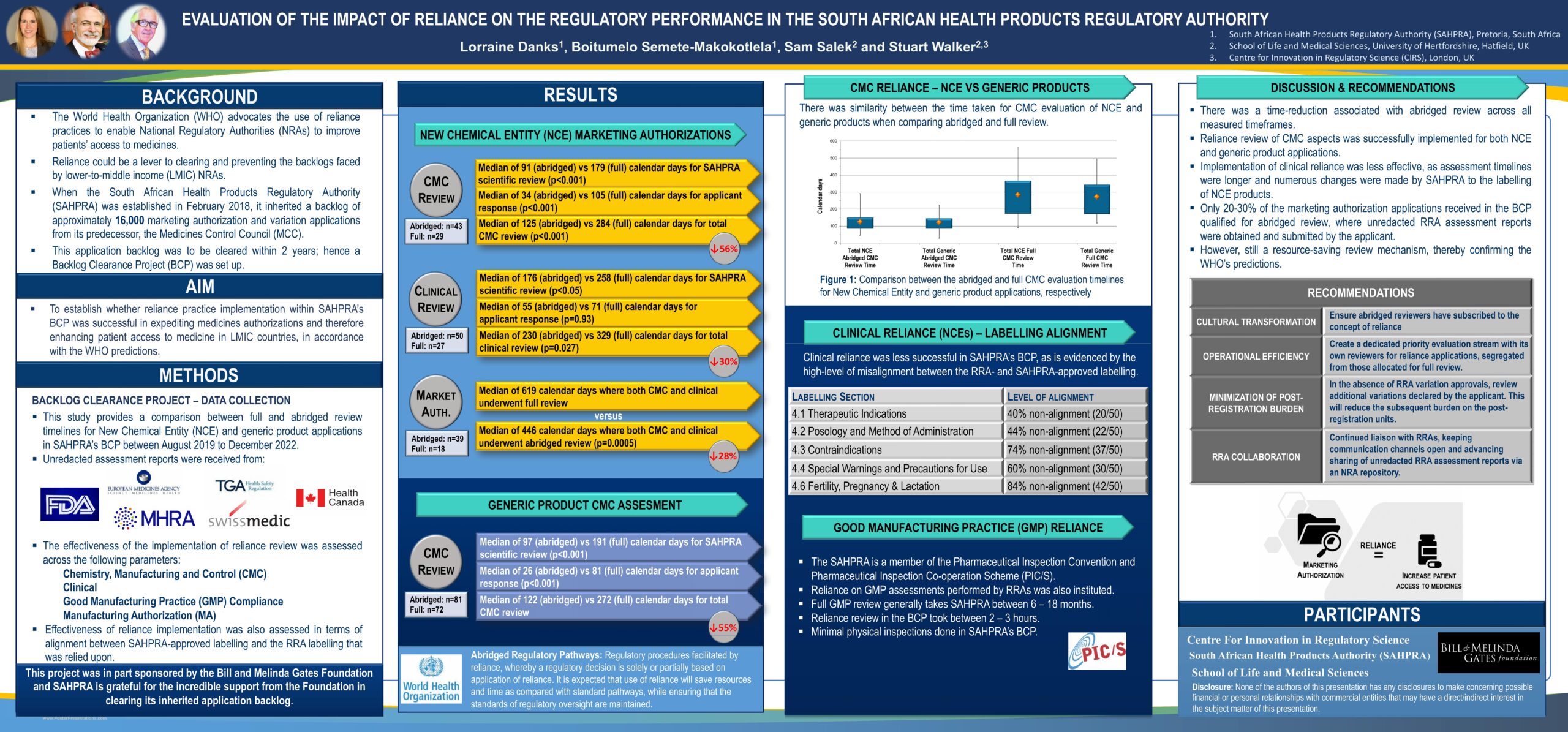
- The World Health Organization (WHO) advocates the use of reliance practices to enable National Regulatory Authorities (NRAs) to improve patients’ access to medicines.
- Reliance could be a lever to clearing and preventing the backlogs faced by lower-to-middle income (LMIC) NRAs.
- When the South African Health Products Regulatory Authority (SAHPRA) was established in February 2018, it inherited a backlog of approximately 16,000 marketing authorisation and variation applications from its predecessor, the Medicines Control Council (MCC).
- This application backlog was to be cleared within 2 years; hence a Backlog Clearance Project (BCP) was set up.
Aims and objectives: To establish whether reliance practice implementation within SAHPRA’s BCP was successful in expediting medicines authorizations and therefore enhancing patient access to medicine in LMIC countries, in accordance with the WHO predictions.
Methods:
- This study provides a comparison between full and abridged review timelines for New Chemical Entity (NCE) and generic product applications in SAHPRA’s BCP between August 2019 to December 2022.
- Unredacted assessment reports were received from US FDA, EMA, Health Canada, TGA, Swissmedic, and MHRA.
- The effectiveness of the implementation of reliance review was assessed across the following parameters: Chemistry, Manufacturing and Control (CMC), Clinical, Good Manufacturing Practice (GMP) Compliance, Manufacturing Authorization (MA)
- Effectiveness of reliance implementation was also assessed in terms of alignment between SAHPRA-approved labelling and the RRA labelling that was relied upon
Discussion:
- There was a time-reduction associated with abridged review across all measured timeframes.
- Reliance review of CMC aspects was successfully implemented for both NCE and generic product applications.
- Implementation of clinical reliance was less effective, as assessment timelines were longer and numerous changes were made by SAHPRA to the labelling of NCE products.
- Only 20-30% of the marketing authorization applications received in the BCP qualified for abridged review, where unredacted RRA assessment reports were obtained and submitted by the applicant.
- However, still a resource-saving review mechanism, thereby confirming the WHO’s predictions.
Recommendations:
- Cultural transformation: Ensure abridged reviewers have subscribed to the concept of reliance.
- Operational efficiency: Create a dedicated priority evaluation stream with its own reviewers for reliance applications, segregated from those allocated for full review.
- Minimization of post-registration burden: In the absence of reference regulatory agency (RRA) variation approvals, review additional variations declared by the applicant. This will reduce the subsequent burden on the post-registration units.
- RRA collaboration: Continued liaison with RRAs, keeping communication channels open and advancing sharing of unredacted RRA assessment reports via an NRA repository.