Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
2023 Workshop Synopsis – Regulatory and reimbursement frameworks for rare disease products
This multi-stakeholder workshop consisted of a series of presentation sessions and three parallel breakout discussions. Presentations explored trends in regulatory and HTA approvals of orphan products and perspectives on incentives …
CIRS RD Briefing 91 – Approaches to Implementing Regulatory Reliance: Considerations for Agencies
This CIRS briefing delves into the increasingly pivotal role of regulatory reliance in the global pharmaceutical landscape. Reliance is defined by World Health Organization (WHO) as the act whereby the …
2023 Workshop report – New ways of working for medicines development
CIRS brought agencies and companies together in a workshop to discuss new ways of working and how the regulatory and HTA landscape in mature and maturing countries should evolve over …
HTA Timelines and Outcomes for MHRA-Approved NASs via Reliance/Work-sharing Routes
During ISPOR Europe 2023 in Copenhagen, Belen Sola presented a poster entitled ‘Study of HTA Timelines and Outcomes for MHRA-Approved NASs in the Post-Brexit UK via Reliance/Work-sharing Routes’. Background: Following …
Evaluation of the Swissmedic regulatory framework for new active substances
Background: Swissmedic is a major regulatory agency that has been benchmarking its timelines for 20 years. To better understand the Swissmedic review times and to examine whether measures introduced to accelerate …
CIRS RD Briefing 90 – Challenges and opportunities for orphan medicines availability in Mexico
The document ‘Estrategia sobre Certidumbre Regulatoria para el Sector Farmacéutico’ (Strategy of Regulatory Certitude for the Pharmaceutical Sector), published last January by COFEPRIS describes important working projects the agency will …
Impact of reliance on the regulatory performance of the South African Health Products Regulatory Authority
Introduction: The World Health Organization (WHO) advocates the use of reliance practices to enable national regulatory authorities (NRAs) to improve patients’ access to medicines. This study considered whether reliance review translates …
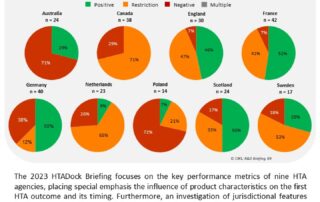
CIRS RD Briefing 89 – Review of HTA outcomes and timelines in Australia, Canada, Europe and the UK 2018-2022
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies, each with unique …
Measuring time to market for new medicines in 7 Asian countries between 2016-21, following review by US FDA or EMA
During the DIA Global Annual Meeting 2023, Adem Kermad, Magda Bujar and Neil McAuslane developed and presented a poster in which they shared the results, recommendations and conclusions about the …
Evaluation of the Effectiveness and Efficiency of Ten Years’ Experience with the East Africa Community Joint Assessment
During the DIA Global Annual Meeting 2023, Nancy Yang-Ngum developed and presented a poster in which she shared the results, recommendations and conclusions about the topic “Evaluation of the Effectiveness …
Evaluation of the Regulatory Review Process of the FDA Ghana: Challenges and Opportunities for Improvement
During the DIA Global Annual Meeting 2023, Mercy Owusu-Asante developed and presented a poster in which she shared the results, recommendations and conclusions about the topic “Evaluation of the Regulatory …
Evaluation of the impact of reliance on the regulatory performance in the South African Health Products Regulatory Authority
During the DIA Global Annual Meeting 2023, Lorraine Danks, Boitumelo Semete-Makokotlela, Sam Salek and Stuart Walker developed and presented a poster in which they shared the results, recommendations and conclusions …
A comparison of the Regional Medicines Regulatory Harmonisation Projects in East, West and Southern Africa.
During the DIA Global Annual Meeting 2023, Tariro Sithole, Nancy Ngum, Mercy Owusu-Asante, Stuart Walker and Sam Salek developed and presented a poster in which they shared the results, recommendations …
A comparison of regulatory decision patterns for oncology products to all other non-oncology products among Swissmedic, EMA and FDA
Consensus of regulatory decisions on the same Marketing Authorization Application (MAA) are critical for stakeholders. In this context, regulatory decision patterns from the Swissmedic (SMC), the US Food and Drug …