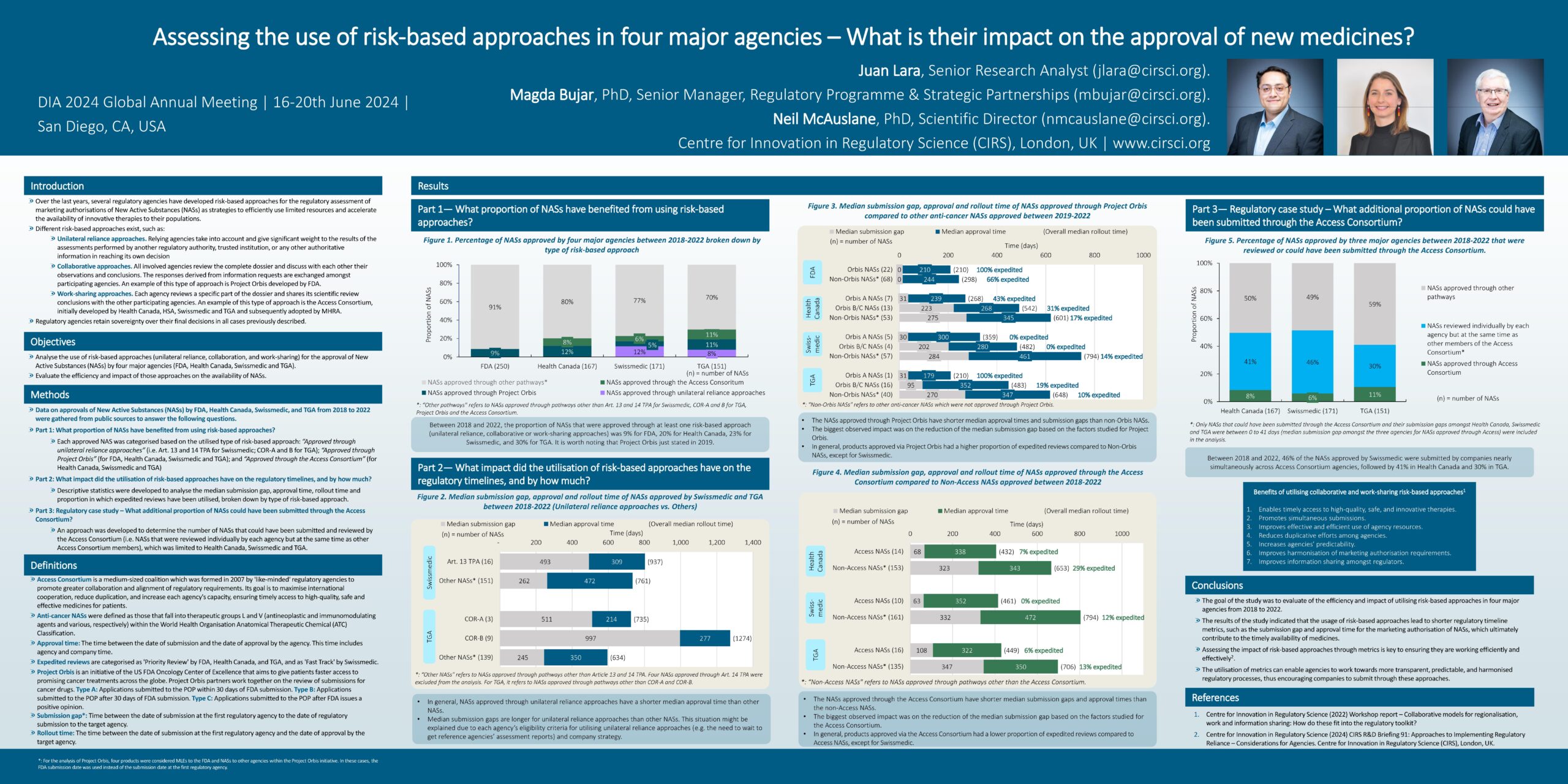
2024 Workshop report – Reliance and regional review models
Faced with increasingly complex technologies and novel evidence generation techniques, regulatory agencies are being challenged to work in new ways. There is pressure on them to be agile and [...]