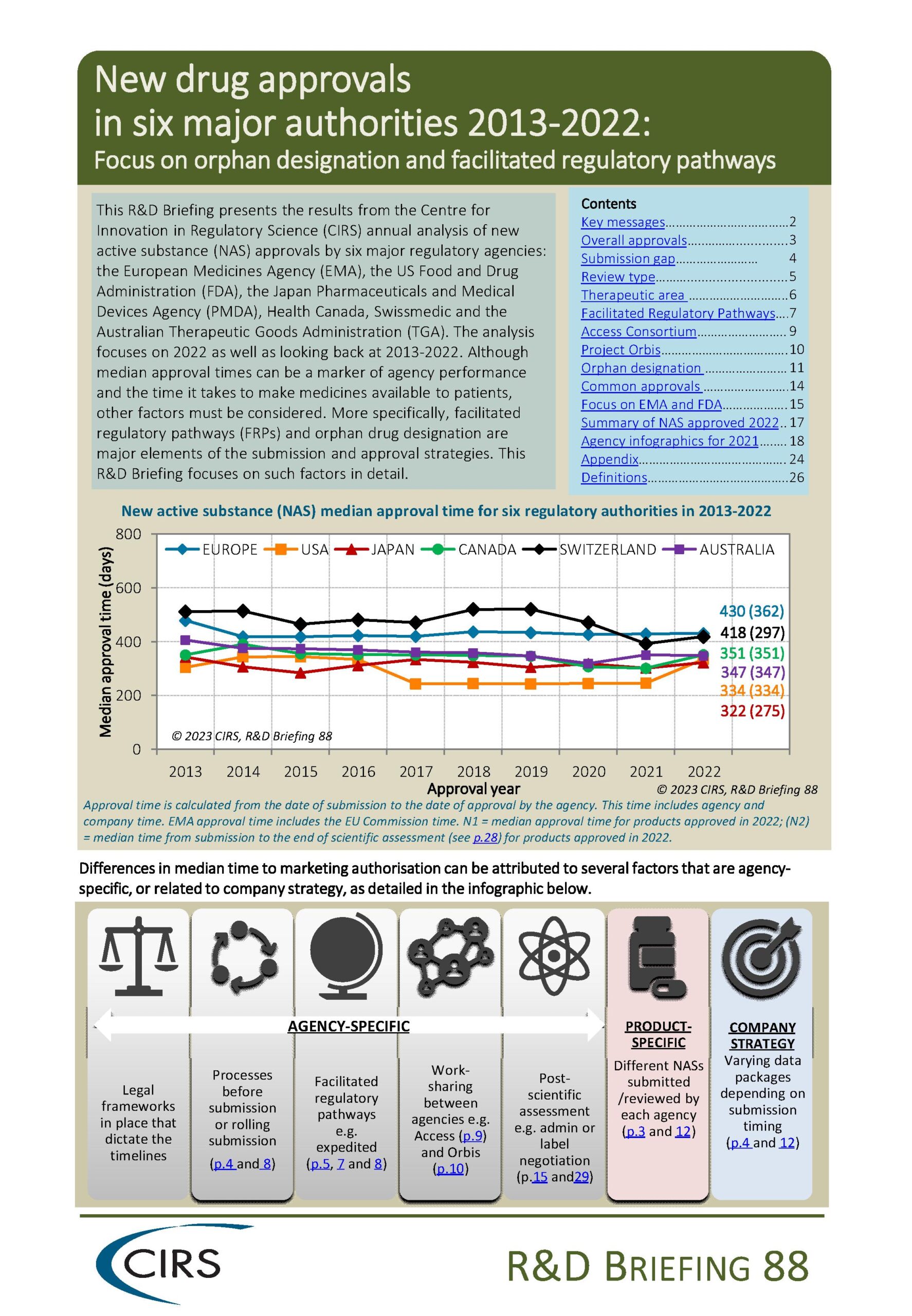
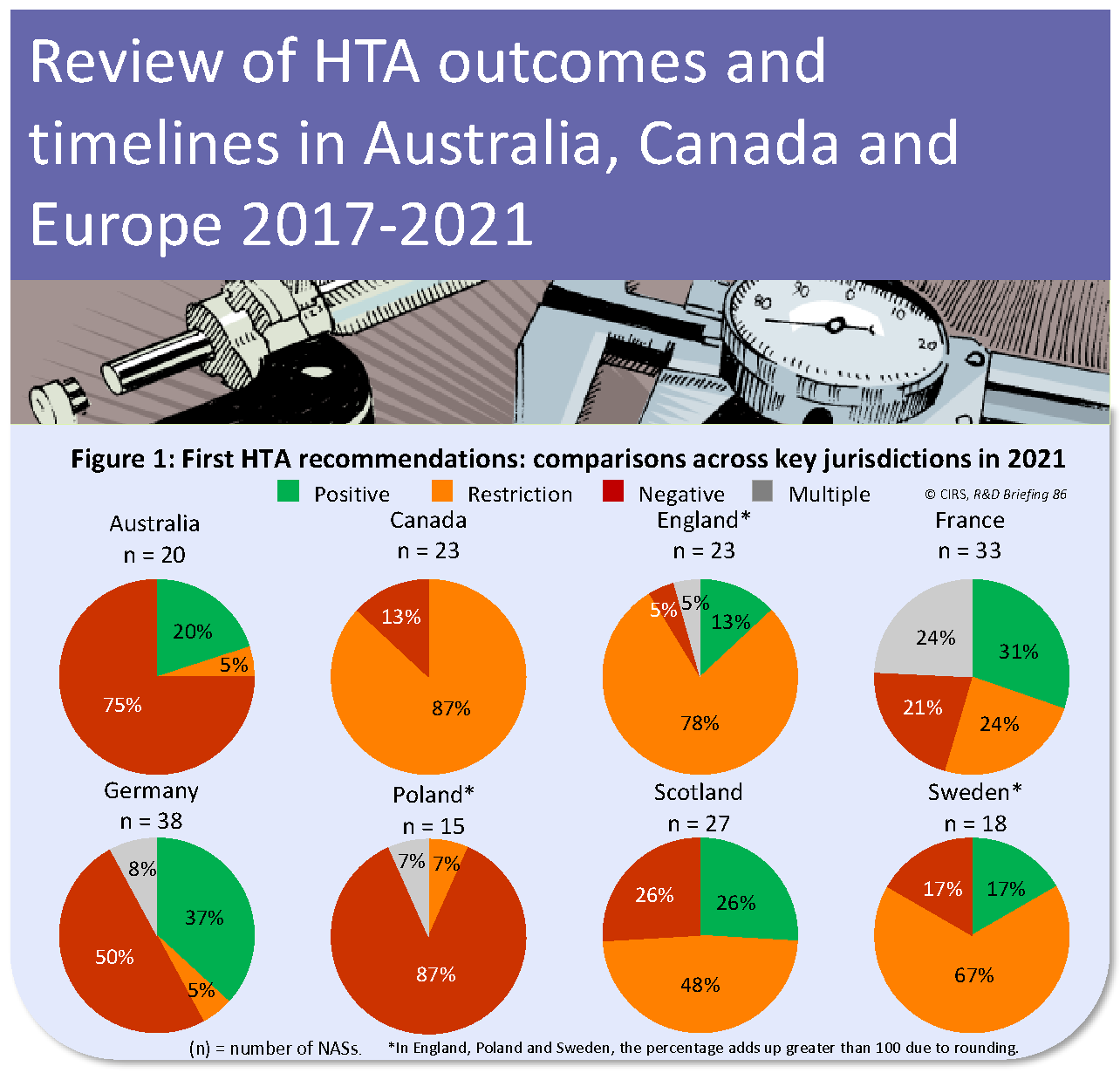
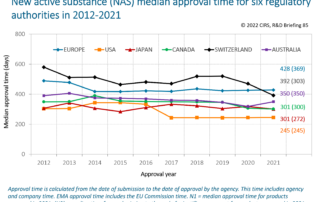
CIRS RD Briefing 88 – New drug approvals in six major authorities 2013-2022: Focus on orphan designation and facilitated regulatory pathways
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the [...]