R&D Briefings
To keep up-to-date with the latest CIRS publications, request to be on our mailing list.
CIRS RD Briefing 91 – Approaches to Implementing Regulatory Reliance: Considerations for Agencies
This CIRS briefing delves into the increasingly pivotal role of regulatory reliance in the global pharmaceutical landscape. Reliance is defined by World Health Organization (WHO) as the act whereby [...]
CIRS RD Briefing 90 – Challenges and opportunities for orphan medicines availability in Mexico
The document ‘Estrategia sobre Certidumbre Regulatoria para el Sector Farmacéutico’ (Strategy of Regulatory Certitude for the Pharmaceutical Sector), published last January by COFEPRIS describes important working projects the agency [...]
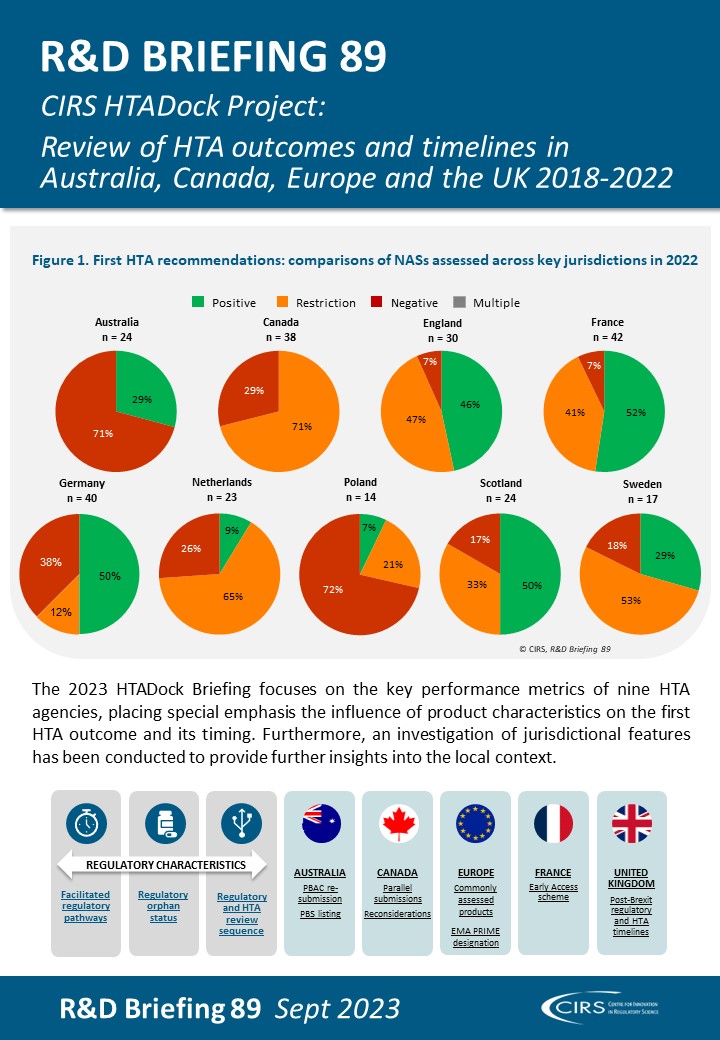
CIRS RD Briefing 89 – Review of HTA outcomes and timelines in Australia, Canada, Europe and the UK 2018-2022
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies, each with [...]
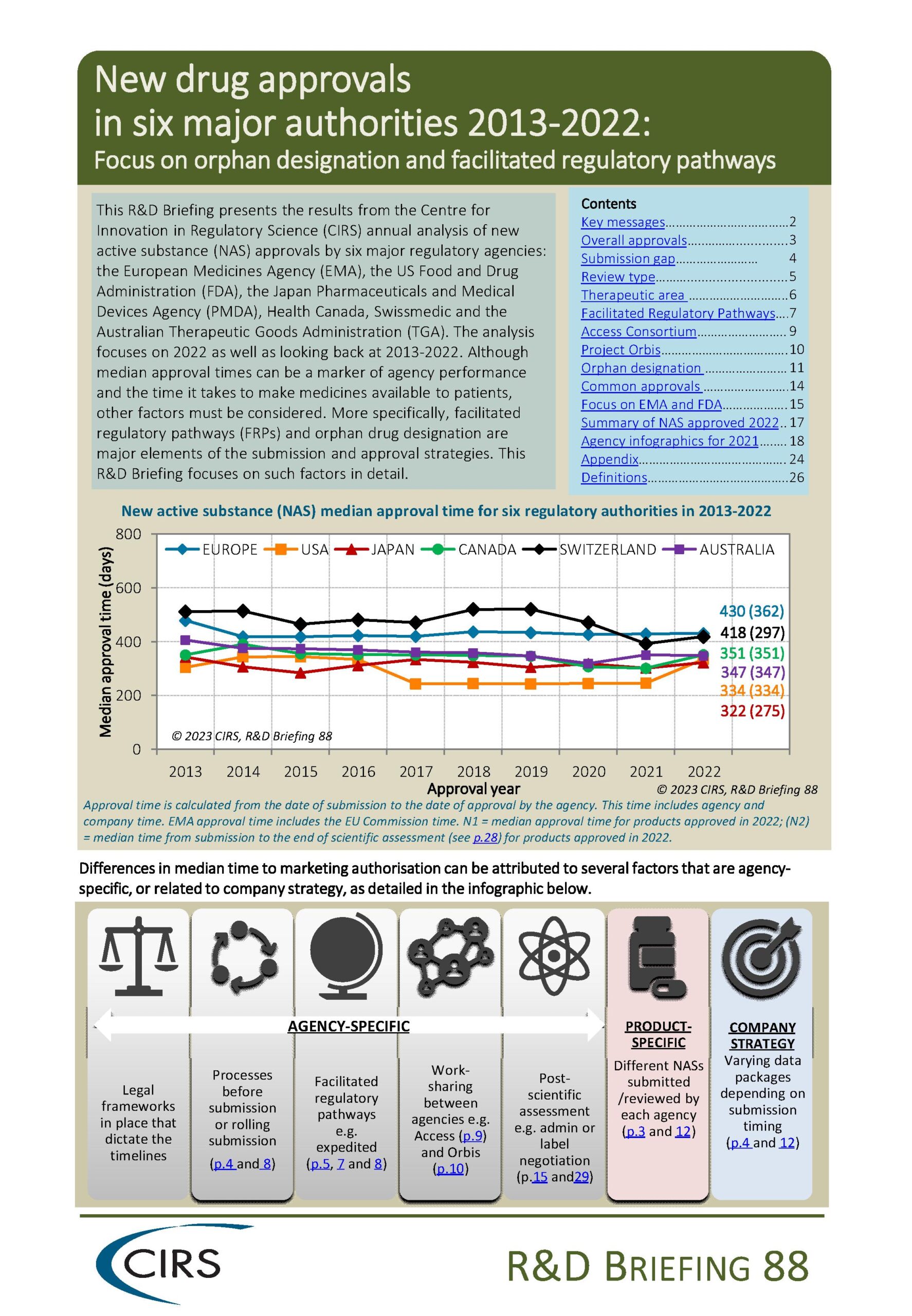
CIRS RD Briefing 88 – New drug approvals in six major authorities 2013-2022: Focus on orphan designation and facilitated regulatory pathways
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the [...]
CIRS RD Briefing 87 – A Roadmap for Regulatory Strengthening: CIRS Tools for Measuring and Optimising Regulatory Performance to Support Practices in Line with the World Health Organization Global Benchmarking Tool Indicators
Over the last 20 years, CIRS has been developing regulatory science tools to increase transparency of processes, support quality regulatory decision making, and provide global advocacy in support of [...]
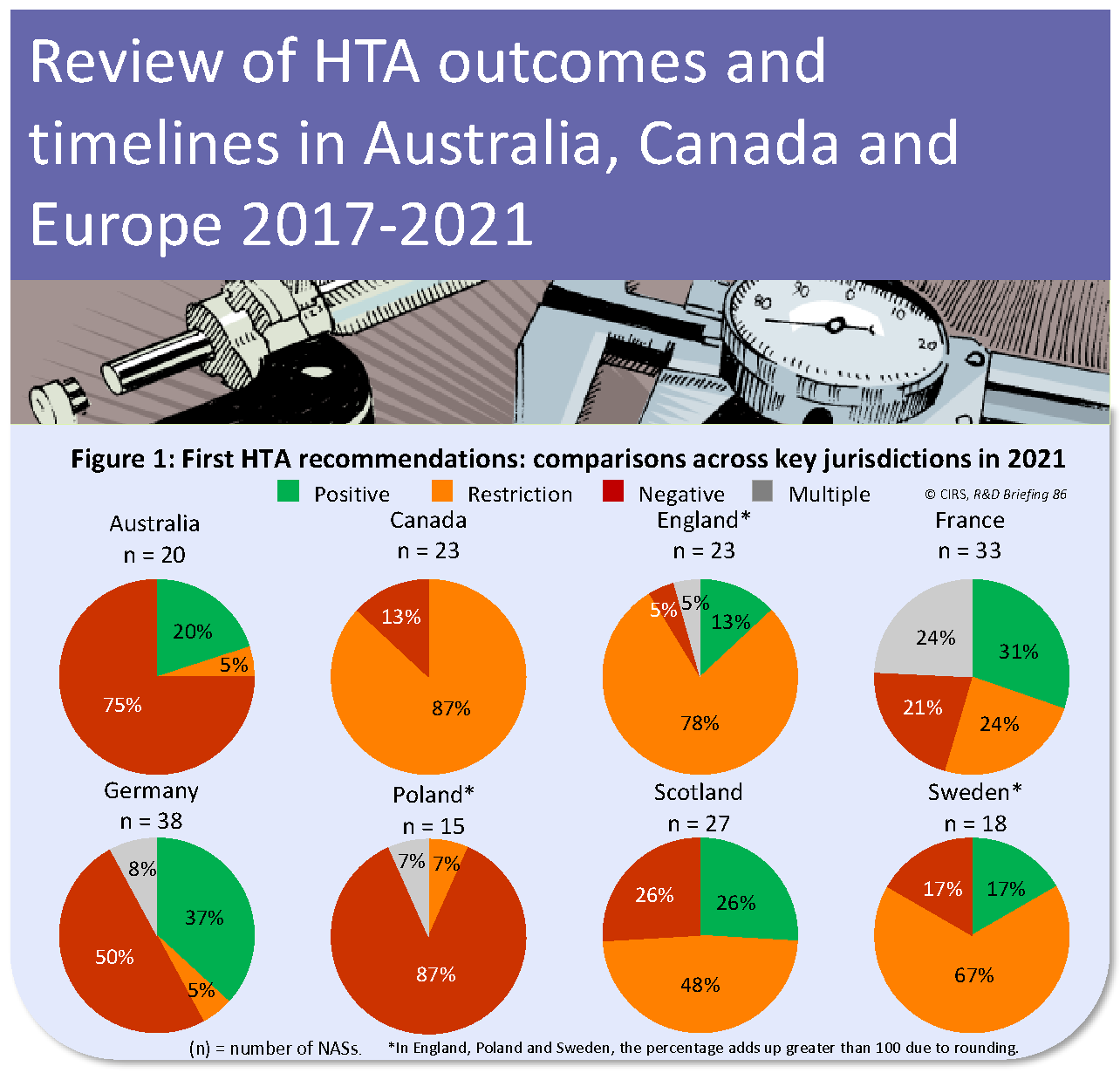
CIRS RD Briefing 86 – Review of HTA outcomes and timelines in Australia, Canada and Europe 2017-2021
The Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory [...]
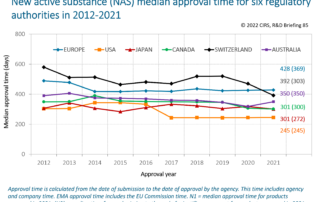
CIRS RD Briefing 85 – New drug approvals in six major authorities 2012-2021
This Briefing presents the results from the CIRS annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food [...]
CIRS RD Briefing 84 – China’s evolving regulatory landscape
China has made significant changes to its medicine regulatory system including: Regulatory reforms - since 2015, regulatory reforms have helped to eliminate application backlogs, improve review timelines and increase [...]
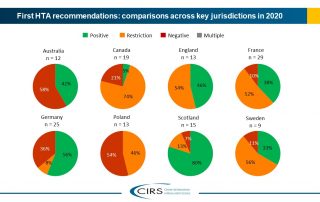
CIRS RD Briefing 83 – HTA outcomes in Australia, Canada and Europe 2016-2020
This Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory [...]
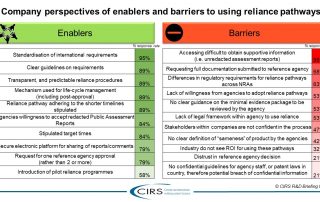
CIRS RD Briefing 82 – Regulatory reliance pathways: opportunities and barriers
An increasing number of National Regulatory Authorities (NRAs) are turning to reliance as a way to conserve resources, build expertise and capacity, increase the quality of their regulatory decisions, [...]