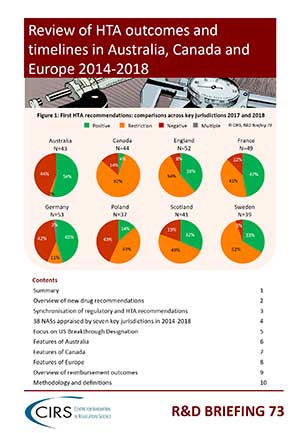
Health technology assessment (HTA)
Bujar et al 2020 – A Process for Evaluating Quality Decision-Making Practices
Background: The development of a medicine is not only underpinned by good science but also by Quality Decision-Making Practices (QDMPs). Indeed, it is important to ensure that all organisations [...]