Health technology assessment (HTA)
CIRS RD Briefing 86 – Review of HTA outcomes and timelines in Australia, Canada and Europe 2017-2021
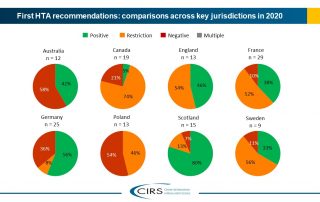
The Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory [...]