Health technology assessment (HTA)
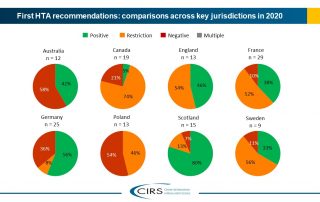
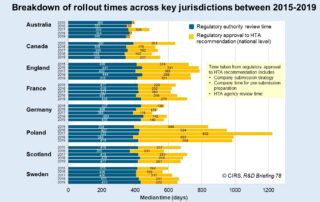
Impact of orphan designation on HTA recommendation time
In recognition of Rare Disease Day 2022, we thought we’d share some insights on the impact of regulatory orphan drug designation on the time to HTA recommendation. Regulatory orphan designation [...]