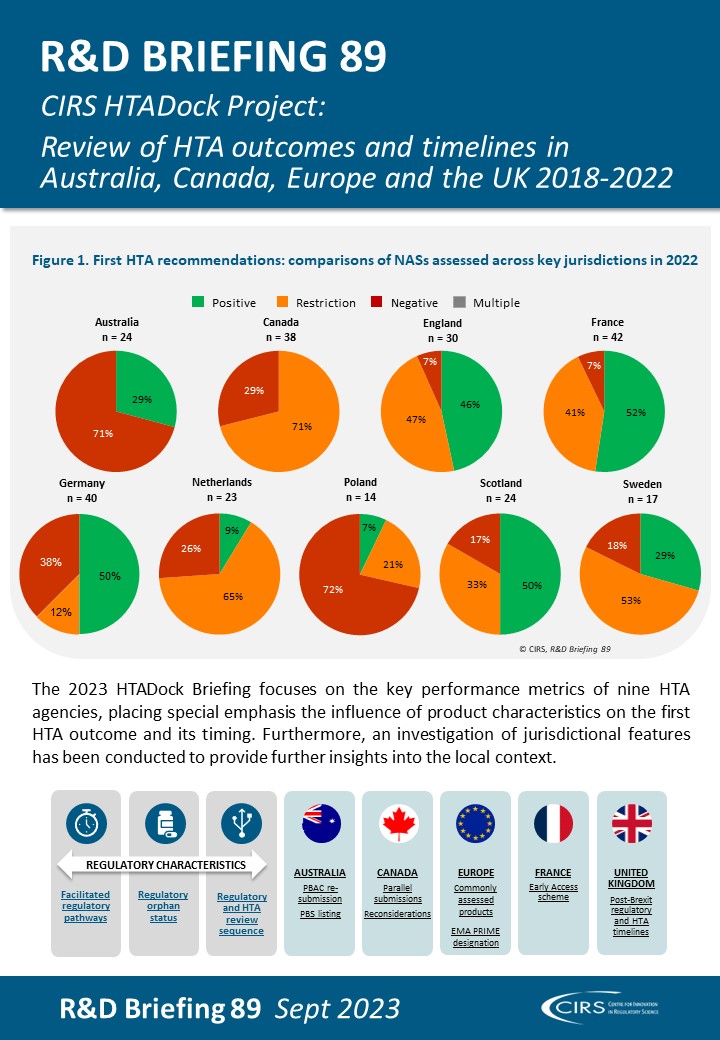
2023 Workshop report – Uncertainty in the development of new medicines
This CIRS workshop brought together companies and agencies (HTA and Regulatory) to discuss the sources of uncertainty that are being built in, by the way medicines development has evolved [...]