R&D Briefings
To keep up-to-date with the latest CIRS publications, request to be on our mailing list.
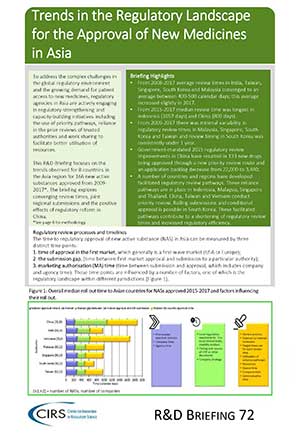
CIRS RD Briefing 72 – Trends in the regulatory landscape Asia
To address the complex challenges in the global regulatory environment and the growing demand for patient access to new medicines, regulatory agencies in Asia are actively engaging in regulatory-strengthening and [...]
CIRS RD Briefing 70 – New approvals in six regulatory authorities 2009-18
Major improvements in the regulatory environment as well as changes in strategies of multinational companies have led to a general decrease in the time to marketing authorisation and improved consistency [...]
CIRS RD Briefing 69: Review of HTA outcomes and timelines 2014-2017
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure that patient access to medicines of therapeutic value is not delayed. As part of [...]
CIRS RD Briefing 68: Regulatory and HTA decision making and access to medicines – the consequences of sequence
Historically, every jurisdiction with some form of regulatory agency capacity has undertaken the review of medicines as a first step in the market access process. This step is intended [...]
CIRS RD Briefing 67 – New approvals in six regulatory authorities 2008-2017
Major improvements in the regulatory environment as well as changes in strategies of multinational companies have led to a decrease in the time to marketing authorisation as well as [...]
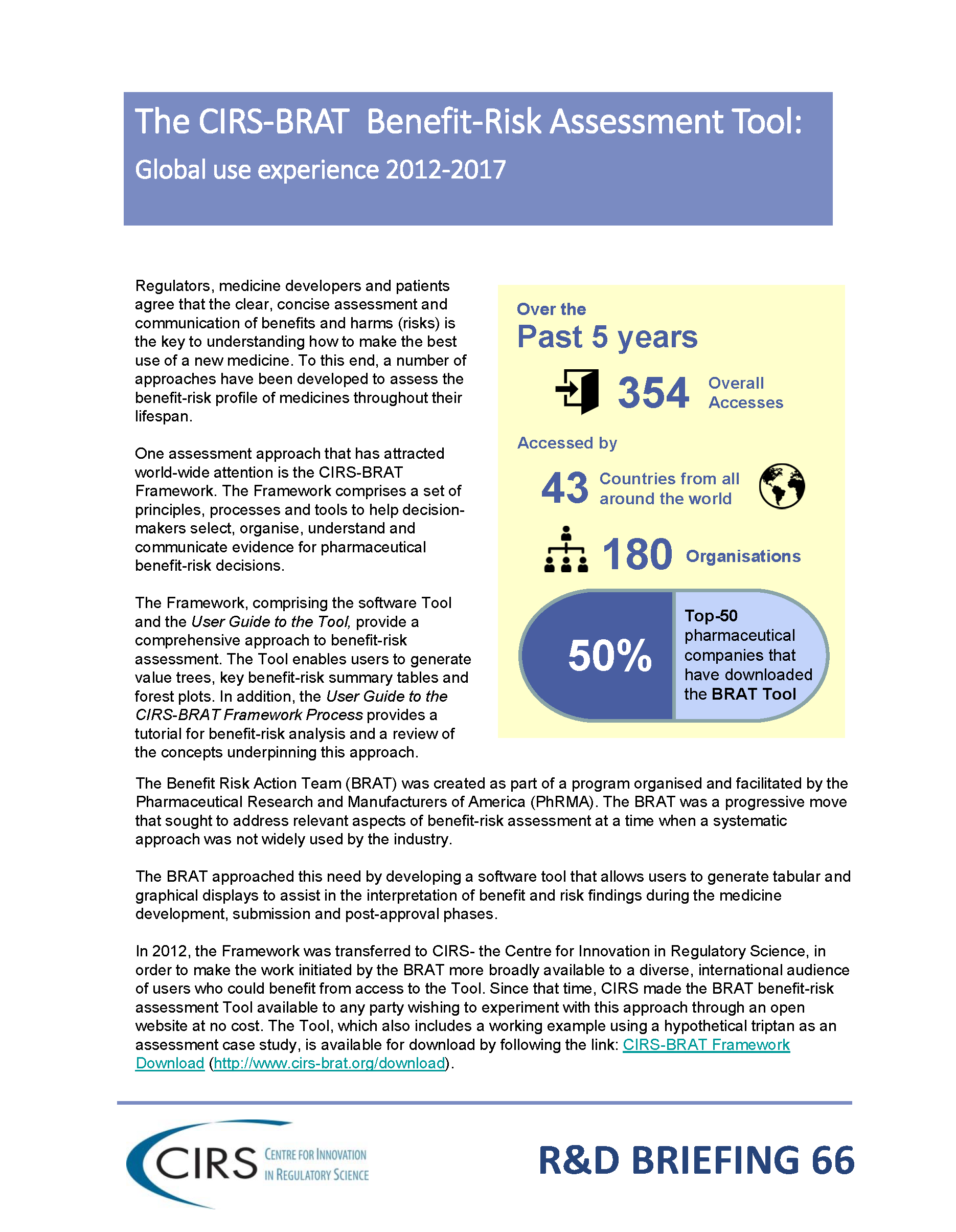
CIRS RD Briefing 66 – Benefit-Risk Assessment Tool (BRAT)
A structured approach to benefit-risk assessment is required as the cornerstone of a consistent way to evaluate and communicate observations regarding a medicine’s benefit-risk profile. The Benefit Risk Action Team [...]
CIRS RD Briefing 65 – New approvals in six regulatory authorities 2007-2016
Over the last decade, 2007-2016, convergence in approval times as well as changes in strategies of multinational pharmaceutical companies have resulted in more new active substances (NASs) being internationalised, [...]
CIRS RD Briefing 64: Review of HTA outcomes and timelines 2014-2015
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure patient access to medicines of therapeutic value. As part of an ongoing study to [...]
CIRS RD Briefing 63: HTA process maps
This R&D Briefing summarises the background and methodology of process mapping with examples to demonstrate the steps involved in regulatory, HTA and coverage processes for new medicines. Process maps [...]
CIRS RD Briefing 62 – New drug approvals in ICH countries 2007-2016
There have been major improvements in the regulatory environment in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) countries over the [...]