Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
Executive Colloquium report – What is the value and return on investment for our company to maintain a regulatory policy function?
In June 2019, the Centre for Innovation in Regulatory Science (CIRS) held an Executive Colloquium in Rockville, MD, USA that brought together representatives from multinational pharmaceutical companies to gauge their …
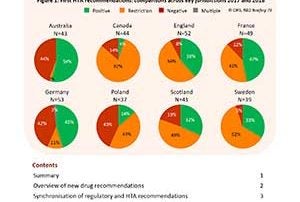
CIRS RD Briefing 73 – HTA outcomes 2014-18
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure that patient access to medicines of therapeutic value is not delayed. As part of an …
CIRS RD Briefing 71 – Trends in the regulatory landscape Latin America
To address the complex challenges in the global regulatory environment and the growing demand for patient access to new medicines, regulatory agencies in Latin America are actively engaging in regulatorystrengthening …
CIRS RD Briefing 72 – Trends in the regulatory landscape Asia
To address the complex challenges in the global regulatory environment and the growing demand for patient access to new medicines, regulatory agencies in Asia are actively engaging in regulatory-strengthening and …
CIRS RD Briefing 70 – New approvals in six regulatory authorities 2009-18
Major improvements in the regulatory environment as well as changes in strategies of multinational companies have led to a general decrease in the time to marketing authorisation and improved consistency …
McAuslane et al 2019 – Confluence of Accelerated Regulatory and HTA Access Pathways
There is a growing interest in aligning accelerated regulatory pathways with flexible access and reimbursement pathways to expedite the equitable availability of high-quality, safe, and effective medicines that provide a …
Keyter et al 2019 – South African Medicines Control Council: Comparison of Its Registration Process
Introduction: Comparisons between regulatory authorities of similar size and regulatory characteristics facilitate value-added benchmarking and provide insight into regulatory performance. Such comparisons highlight areas for improvement as authorities move toward …
Bujar et al 2019 – Quality decision making in Health Technology Assessment
Background: To evaluate the quality of the decision-making processes of pharmaceutical companies during medicines development for evidence generation to support reimbursement of new medicines and the appraisal recommendation decision-making process …
Bujar et al 2019 – Reliability and relevance of a decision-making instrument
Introduction: The Quality of Decision-Making Orientation Scheme (QoDoS) was developed to provide organisations involved in submission, approval and reimbursement of new medicines with a tool to improve the quality of …
2018 Forum report – Managing uncertainties for products using early access pathways
In November 2018, a collaborative forum presented by CIRS and the Utrecht University WHO Collaborating Centre for Pharmaceutical Policy and Regulation brought together regulatory, HTA, industry and academic perspectives with …
2017 Workshop report – Flexible regulatory/access pathways
The aim of this workshop was to bring together companies, patient representatives and HTA, payer and regulatory agencies to discuss current perspectives and opportunities for Facilitated Regulatory Pathways (FRPs) and …
CIRS RD Briefing 69: Review of HTA outcomes and timelines 2014-2017
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure that patient access to medicines of therapeutic value is not delayed. As part of an …
CIRS RD Briefing 68: Regulatory and HTA decision making and access to medicines – the consequences of sequence
Historically, every jurisdiction with some form of regulatory agency capacity has undertaken the review of medicines as a first step in the market access process. This step is intended to …
Wang et al 2018 – Building Synergy between Regulatory and HTA Agencies
Objectives: To evaluate the current practice of companies and agencies to assess the changes made in aligning regulatory and health technology assessment (HTA) stakeholders; to identify areas of commonality of …
2018 Workshop report – Practical implementation of reliance models
This Workshop was a follow-on from that held in 2017 in Sao Paulo entitled “Facilitating the review of new medicines through risk-based evaluations: How can a stratification process be utilised …