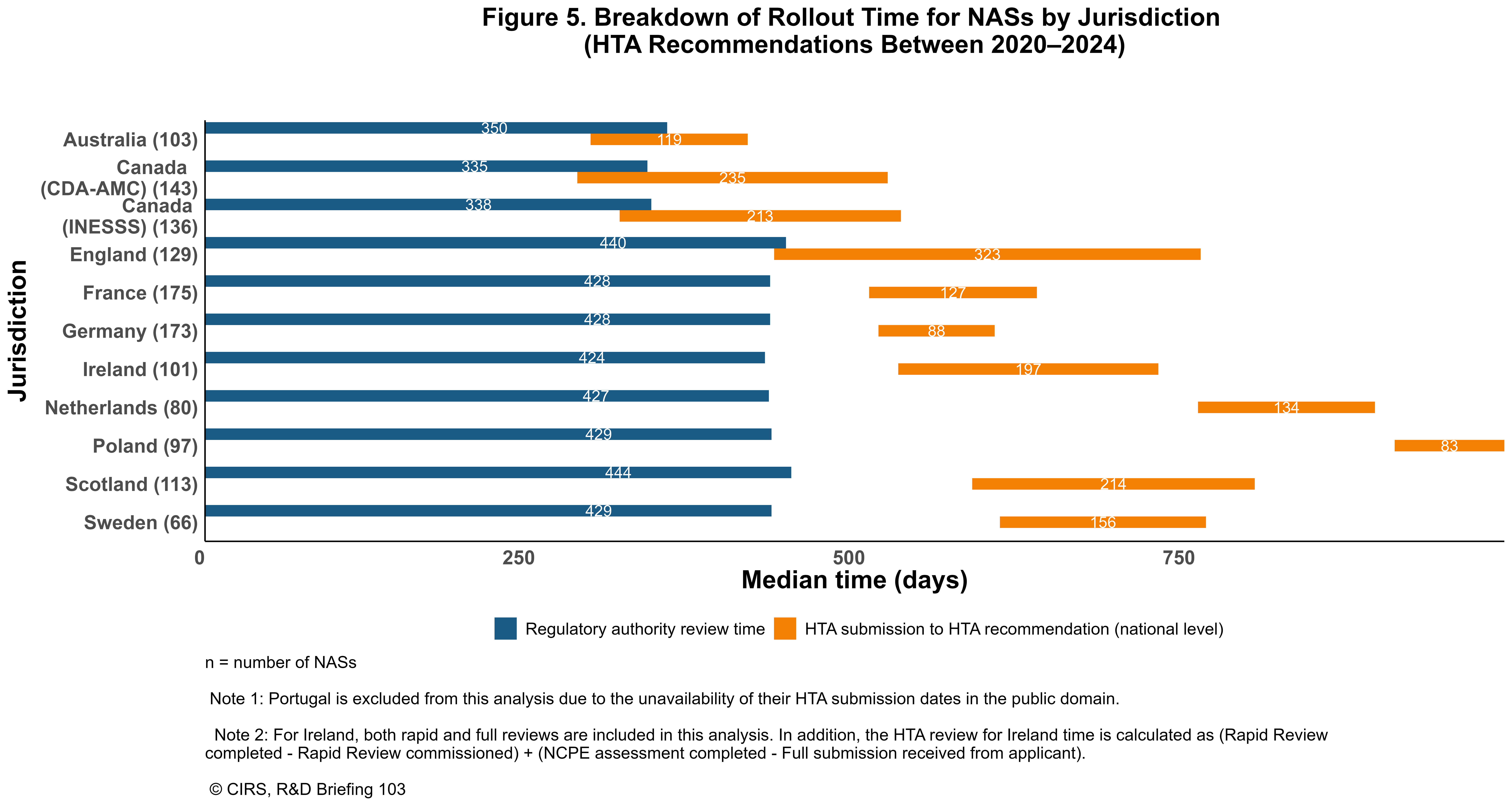
CIRS RD Briefing 103 – Review of HTA outcomes and timelines in Australia, Canada, Europe and the UK, 2020-2024
This R&D Briefing presents findings from HTADock, an ongoing CIRS metrics study that collects publicly available data on new active substances (NASs) appraised by 12 HTA agencies in Australia [...]