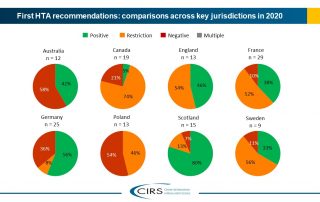
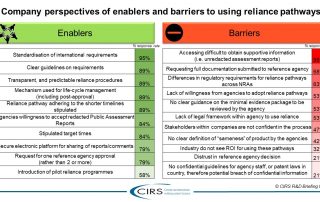
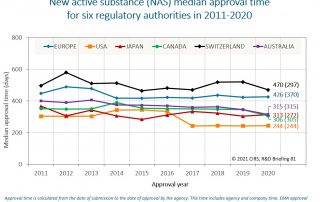
Good Review Practices in the Southern African Development Community
Introduction: National medicines regulatory agencies are faced with challenges including limited resources and technical capacity, resulting in countries collaborating and sharing resources to improve efficiency of the review process to [...]