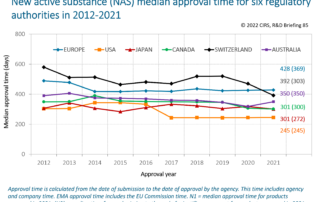
Regulatory, HTA and company interactions: the current landscape and future ecosystem for drug development, review and reimbursement
Background: Multi-stakeholder interactions have evolved at product and policy levels. There is a need to assess the current and future landscape of interactions between companies, and regulatory and HTA agencies [...]