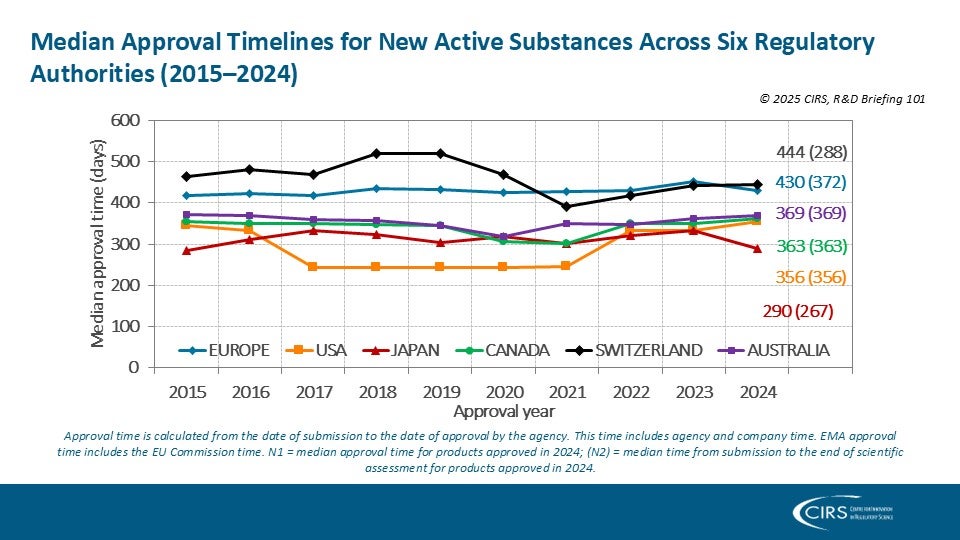
Evaluation of good review practices at FDA Ghana – Owusu-Asante 2025
The aim of this study was to assess the good review practices (GRevPs) of the Food and Drugs Authority (FDA) Ghana in order to identify opportunities for improvement. Reviewers [...]