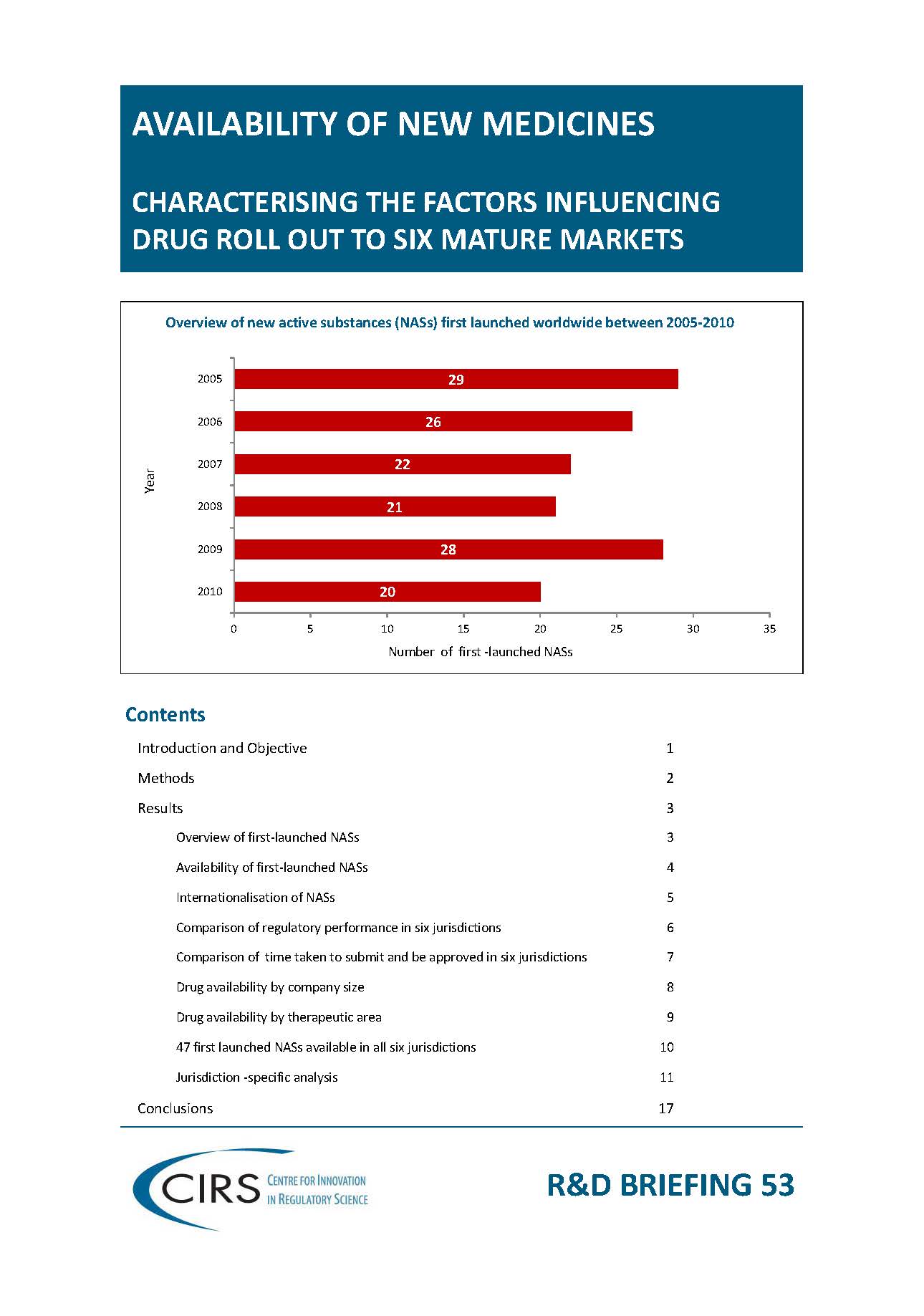
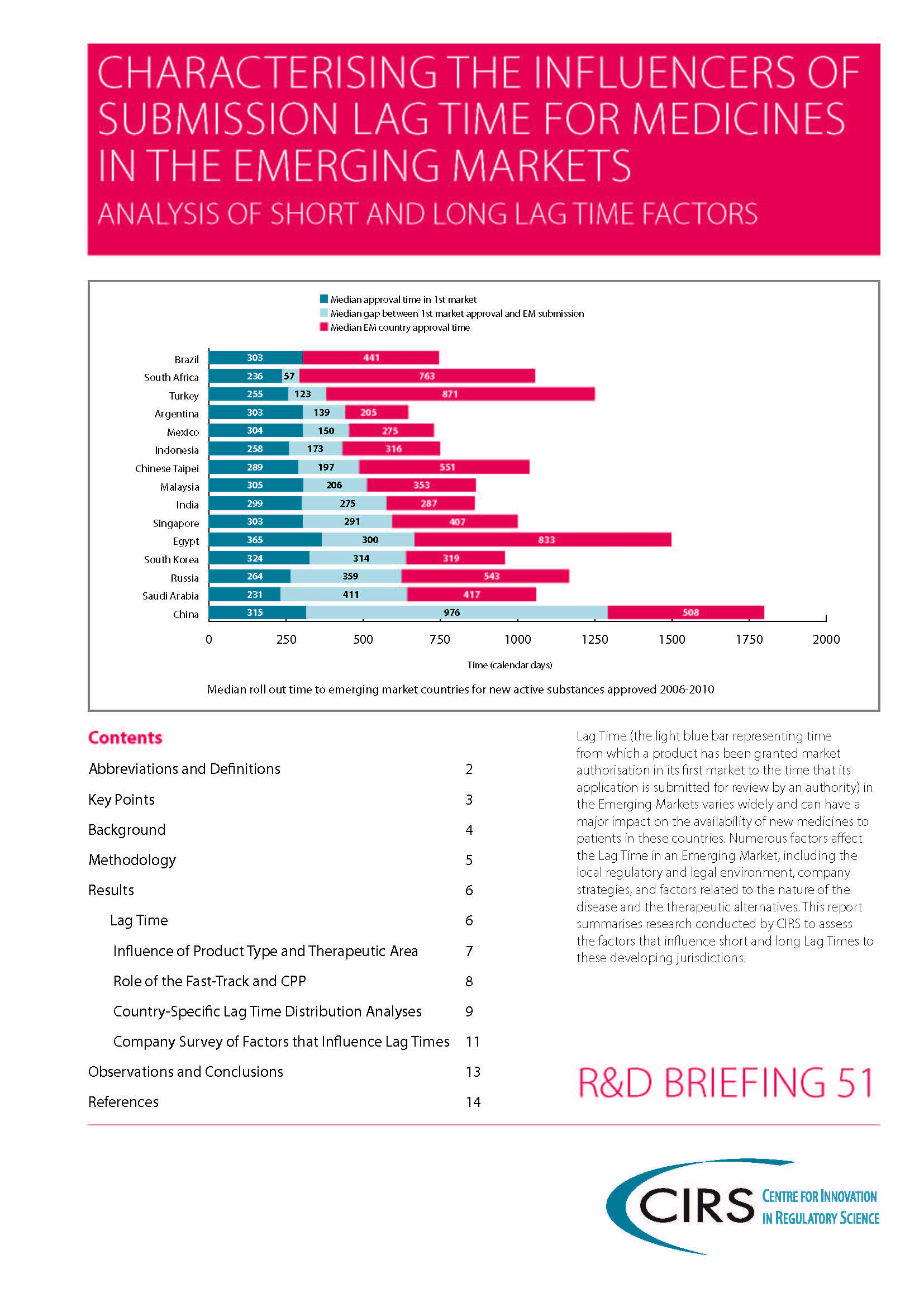
2014 Workshop report – Focus on Latin America: Building quality submission and review processes and practices
Focus on Latin America: Building quality submission and review processes and practices - Overcoming challenges and meeting expectations 23-24 January 2014, Lima, Peru This workshop was held to discuss [...]