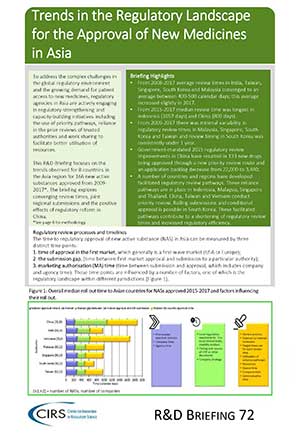
Keyter et al 2019 – Evaluation of the performance of the South African regulatory agency
Background: Timely access to new medicines may be addressed through strengthening of registration efciencies and timelines by establishing and refning value-added registration processes, resources, and systems. The aims of [...]