Health technology assessment (HTA)
Canadian HTA landscape: Comparing HTA timelines and outcomes for CDA-AMC and INESSS
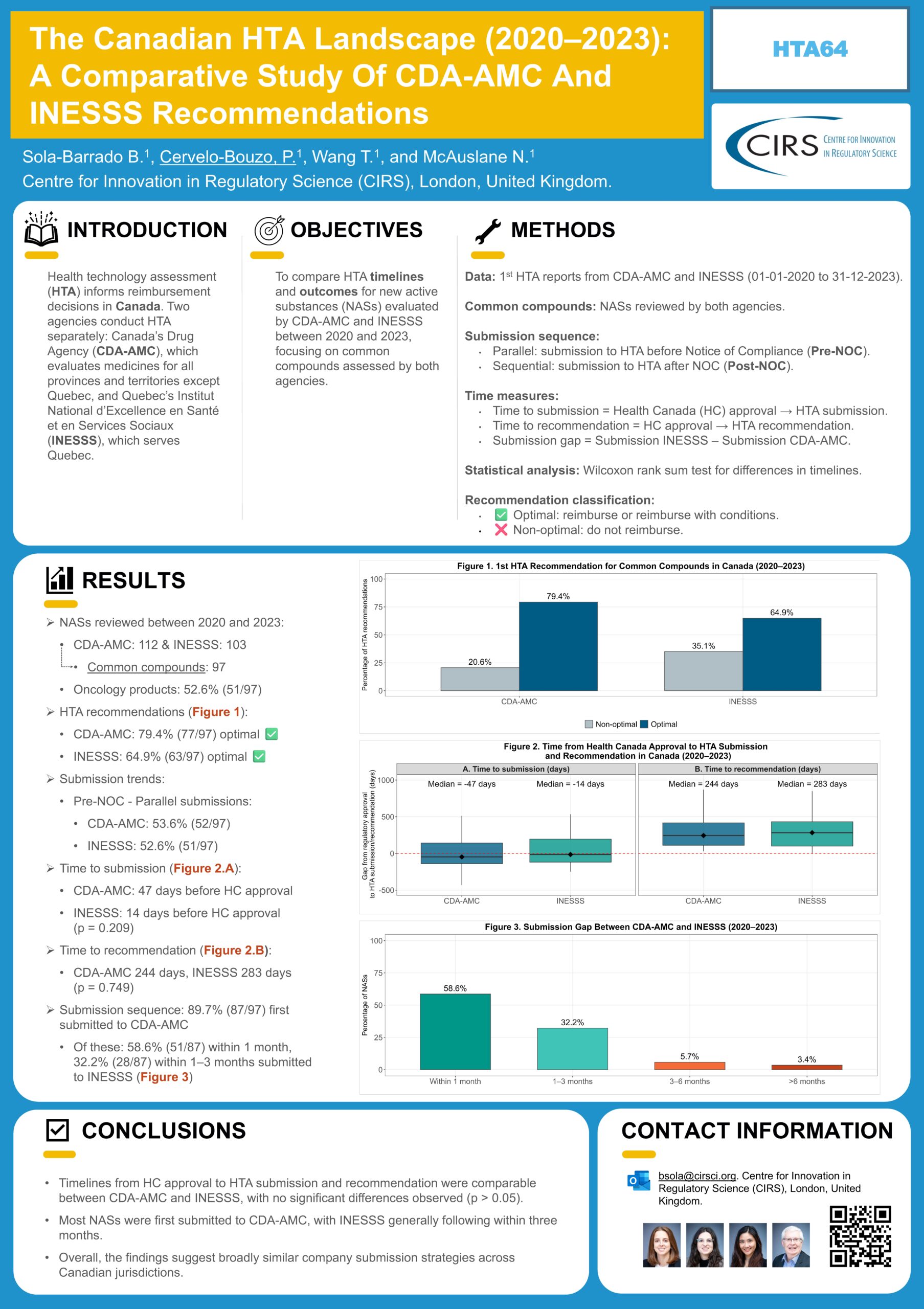
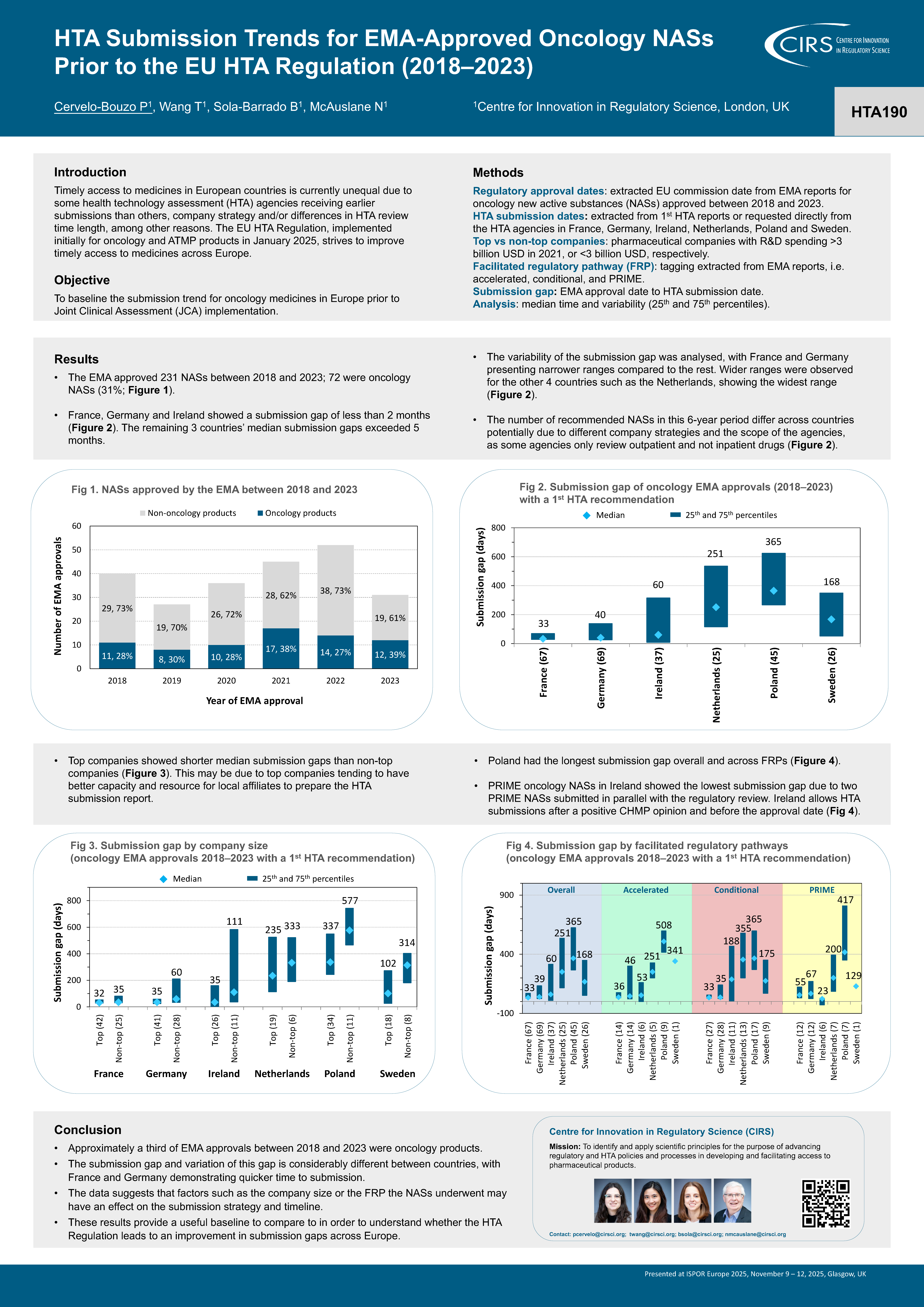
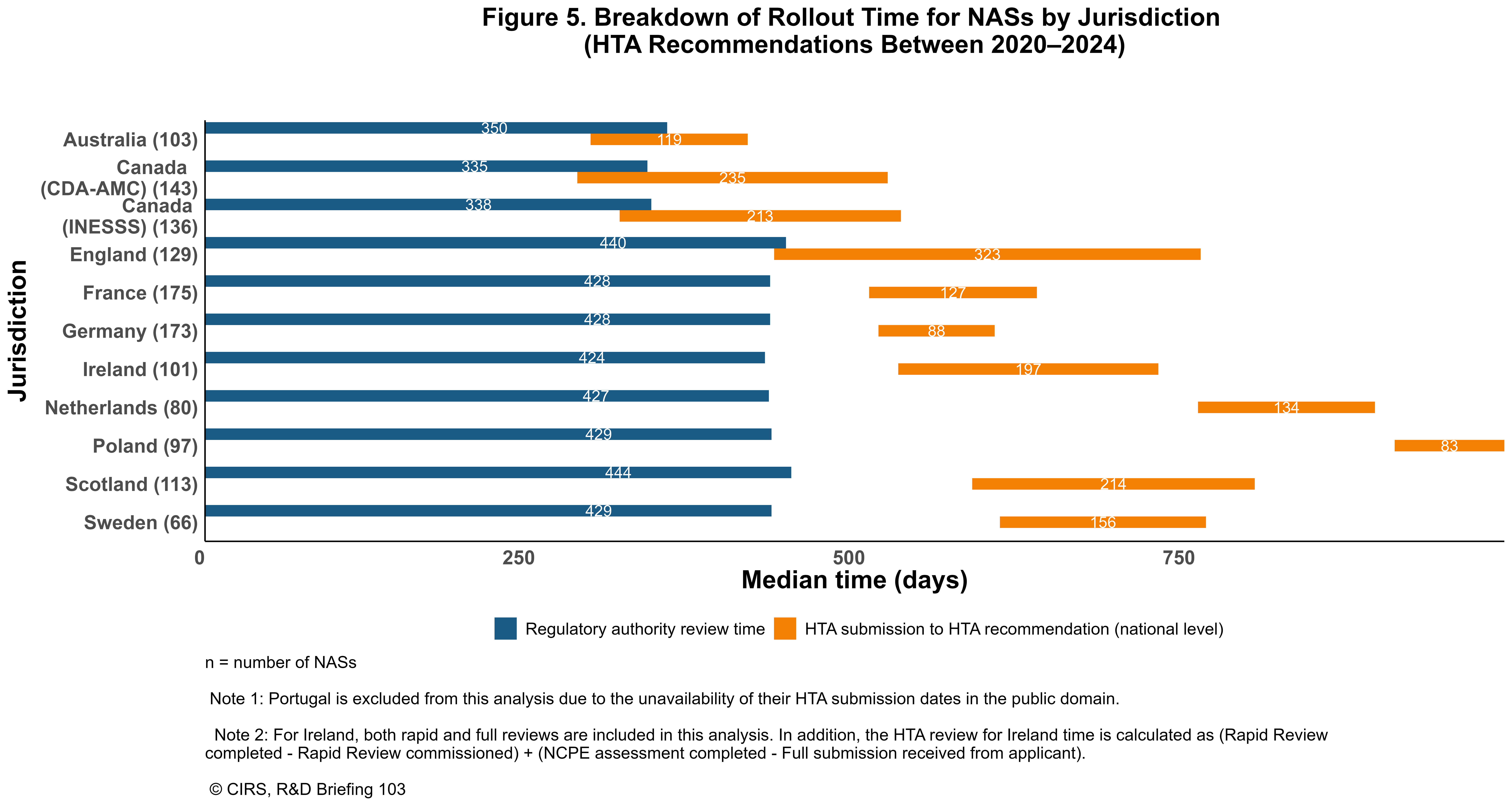
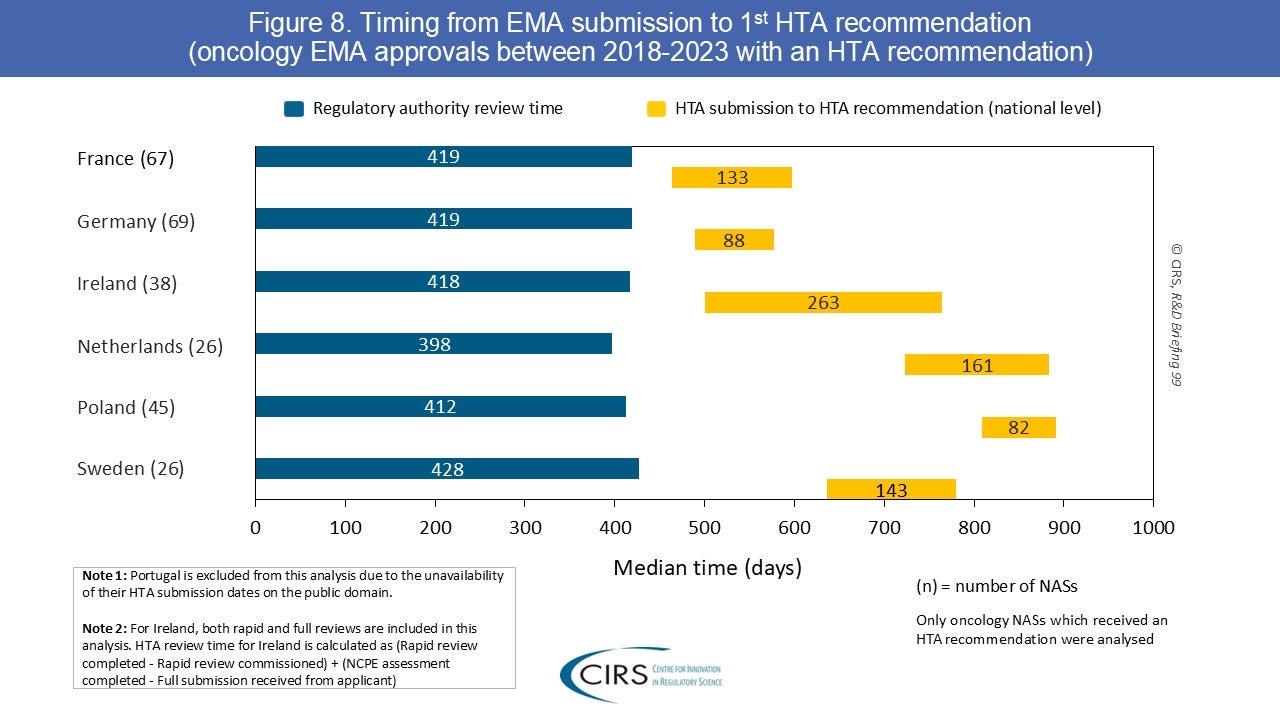
How do HTA timelines and recommendations compare across Canada’s HTA agencies? At ISPOR Europe 2025, we presented a poster analysing HTA timelines and outcomes for Canada's Drug Agency (CDA‑AMC) [...]