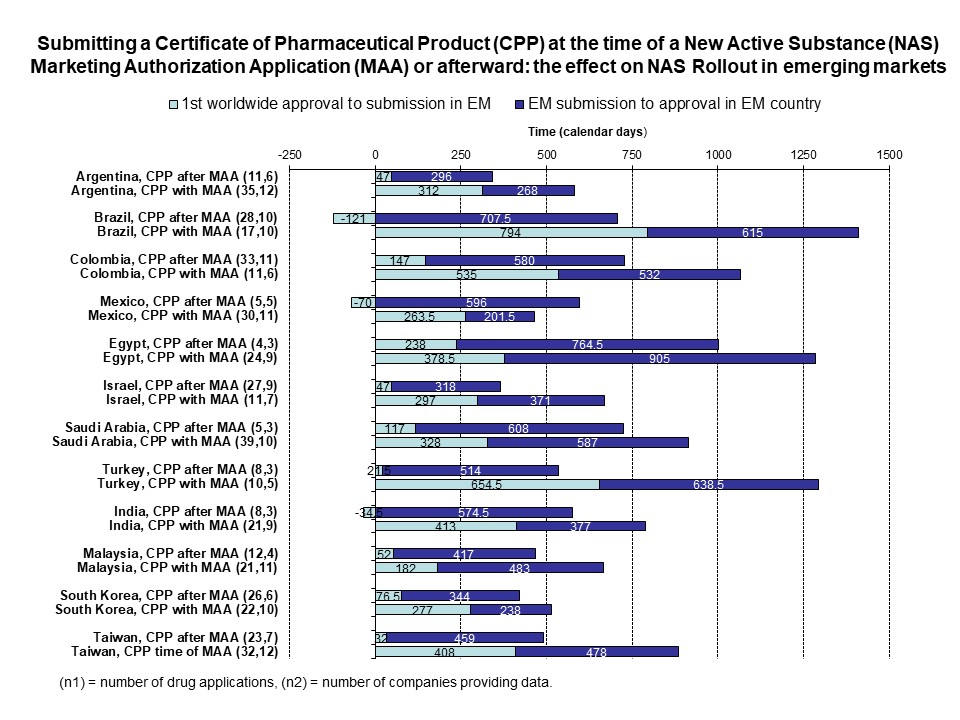
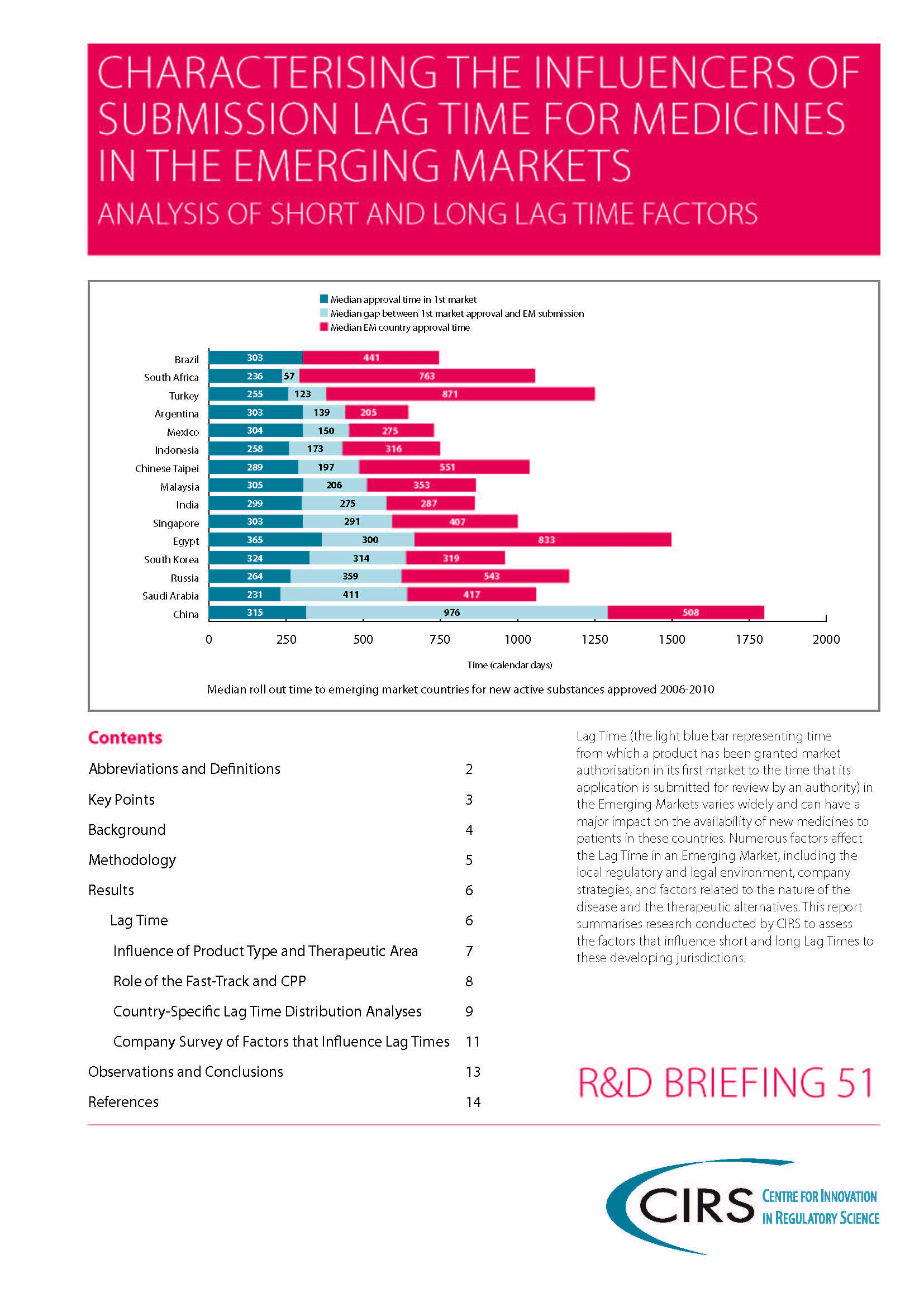
Rodier et al 2020 – Use of the CPP in 18 maturing markets
Background: The certificate of pharmaceutical product (CPP) was implemented to accelerate the availability of new drugs in developing countries by providing evidence of the quality of products and reducing [...]