R&D Briefings
To keep up-to-date with the latest CIRS publications, request to be on our mailing list.
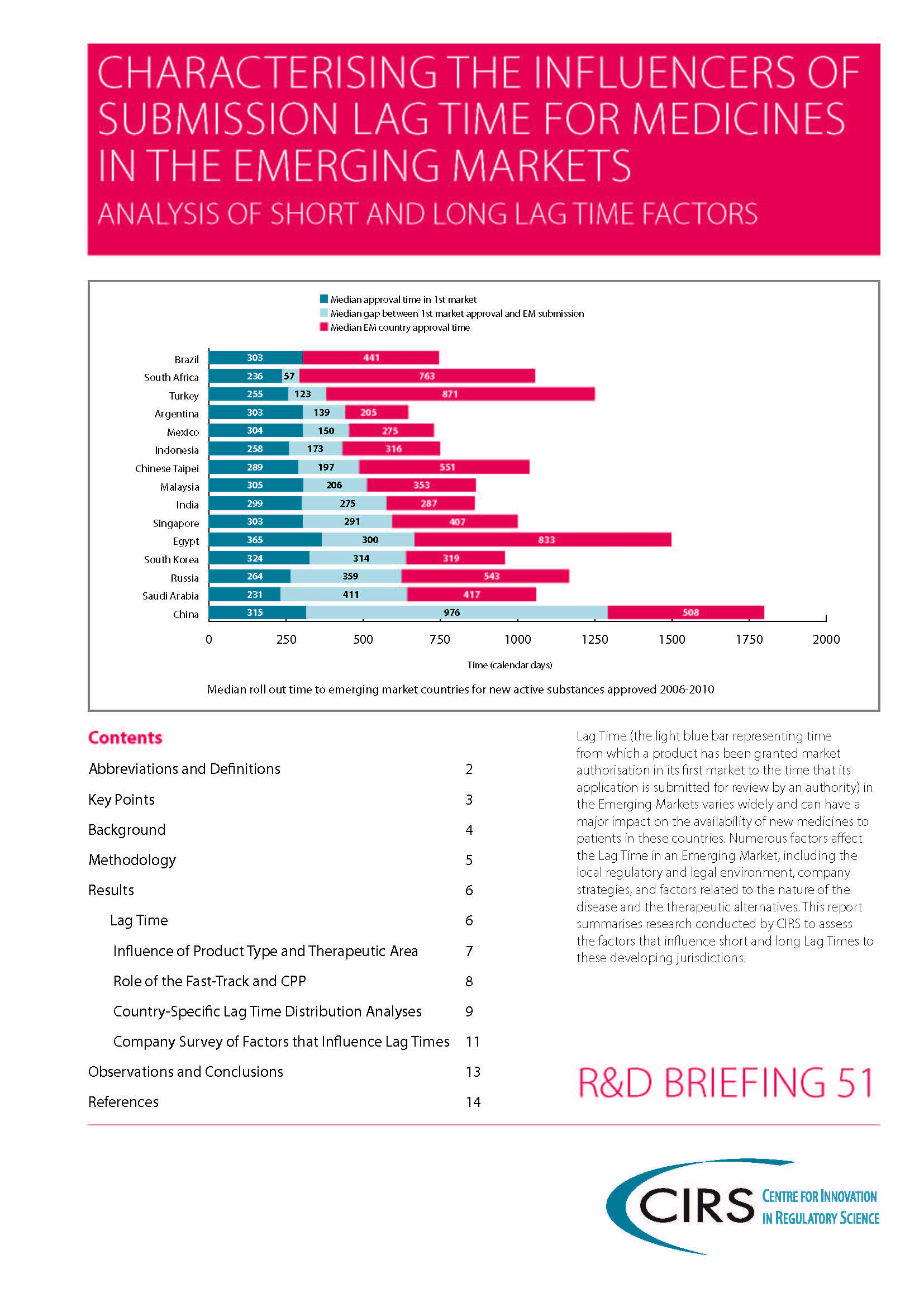
CIRS RD Briefing 51 – Submission lag time in the emerging markets
Lag Time (the time from which a product has been granted market authorisation in its first market to the time that its application is submitted for review by an [...]
CIRS RD Briefing 51 – New drug approvals in ICH countries 2002-2011
In 2011, the number of New Active Substances (NASs) approved in all the ICH countries increased compared to 2010, and FDA represented the largest number of new medicines approved [...]
CIRS RD Briefing 46: Building quality into regulatory activities
The Institute for Regulatory Science is currently involved in several activities related to quality as it applies to regulatory submissions and procedures, rather than the more conventional association with [...]
CIRS RD Briefing 50 – Cross-regional comparison of regulatory environment in emerging markets
The emerging markets of Asia-Pacific, the Middle East, Africa and Latin America are becoming increasingly important to pharmaceutical companies in their global strategies for the registration of new medicines [...]
CIRS RD Briefing 49 – Accessing the regulatory environment – Latin America
The Regulatory Agencies in Latin America share a common goal with the research-based pharmaceutical companies that they regulate. This is to ensure that new medicines become available to patients [...]
CIRS RD Briefing 48 – Assessing the regulatory environment – Middle East and Africa
The regulatory agencies in the Middle East and African region that were included in this study share a common goal with the research-based pharmaceutical companies that they regulate. This [...]
CIRS RD Briefing 47 – Accessing the regulatory environment – SE Asia and Western Pacific
The regulatory agencies in South East Asia and the Western Pacific share a common goal with the research-based pharmaceutical companies that they regulate. This is to ensure that new [...]
CIRS RD Briefing 44: Global drug development and regulatory review
Although it is the goal of most major pharmaceutical companies to achieve global development for new medicines with simultaneous submission of the regulatory dossier in the three main ICH [...]
CIRS RD Briefing 43: Current strategies in global drug development
A summary of the outcome of a survey carried out by the CMR International Institute for Regulatory Science among pharmaceutical companies. Although the pharmaceutical industry is moving towards integrated [...]
CIRS RD Briefing 45: Regulatory timelines in Saudi Arabia
This unique study is the first in-depth analysis of the trends and changes in the regulatory approval times for medicines in the Kingdom of Saudi Arabia. Not only have [...]