R&D Briefings
To keep up-to-date with the latest CIRS publications, request to be on our mailing list.
CIRS RD Briefing 61: Building quality into decision-making processes
In 2015, CIRS initiated a programme in Quality Decision Making with the following aims: Evaluate the current decision-frameworks and understand the characteristics of different decision-making processes Assess the quality [...]
CIRS RD Briefing 60 – Early scientific advice from HTA agencies
This R&D Briefing 60 summarises highlights from the Technical Forum convened by CIRS on 11 December 2015, Heathrow, UK. Forum objectives: Identify companies’ current approaches to seeking early scientific [...]
CIRS RD Briefing 59 – New approvals in six regulatory authorities 2006-2015
The last decade, 2006-2015, has seen a continuation of the convergence and general decrease in the approval times amongst six major regulatory authorities, namely the European Medicines Agency (EMA), [...]
CIRS RD Briefing 58: Changing regulatory environment in Latin America
The aim of this Briefing is to review and summarise the findings from the major studies and interactions carried out by CIRS in LATAM in the last decade in [...]
CIRS RD Briefing 57 – New drug approvals in ICH countries 2005-2014
There have been major improvements in the regulatory environment in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) countries over the [...]
CIRS RD Briefing 56: Understanding the dynamics of China’s regulatory environment
Changes have occurred in the organisation and procedural activities of the China Food and Drug Administration (CFDA) and the Centre for Drug Evaluation (CDE). Initiatives that were designed to [...]
CIRS RD Briefing 55 – Approvals across six major authorities 2004-2013
As part of the ongoing study to monitor regulatory performance, CIRS has analysed the trends in new medicines’ approval between 2004 and 2013 by six regulatory authorities including Health [...]
CIRS RD Briefing 54 – Approvals in ICH countries 2004-2013
In 2013, the overall number of New Active Substances (NASs) approved by EMA, FDA and PMDA was comparable across the three agencies. Nevertheless, despite this similarity, the number of [...]
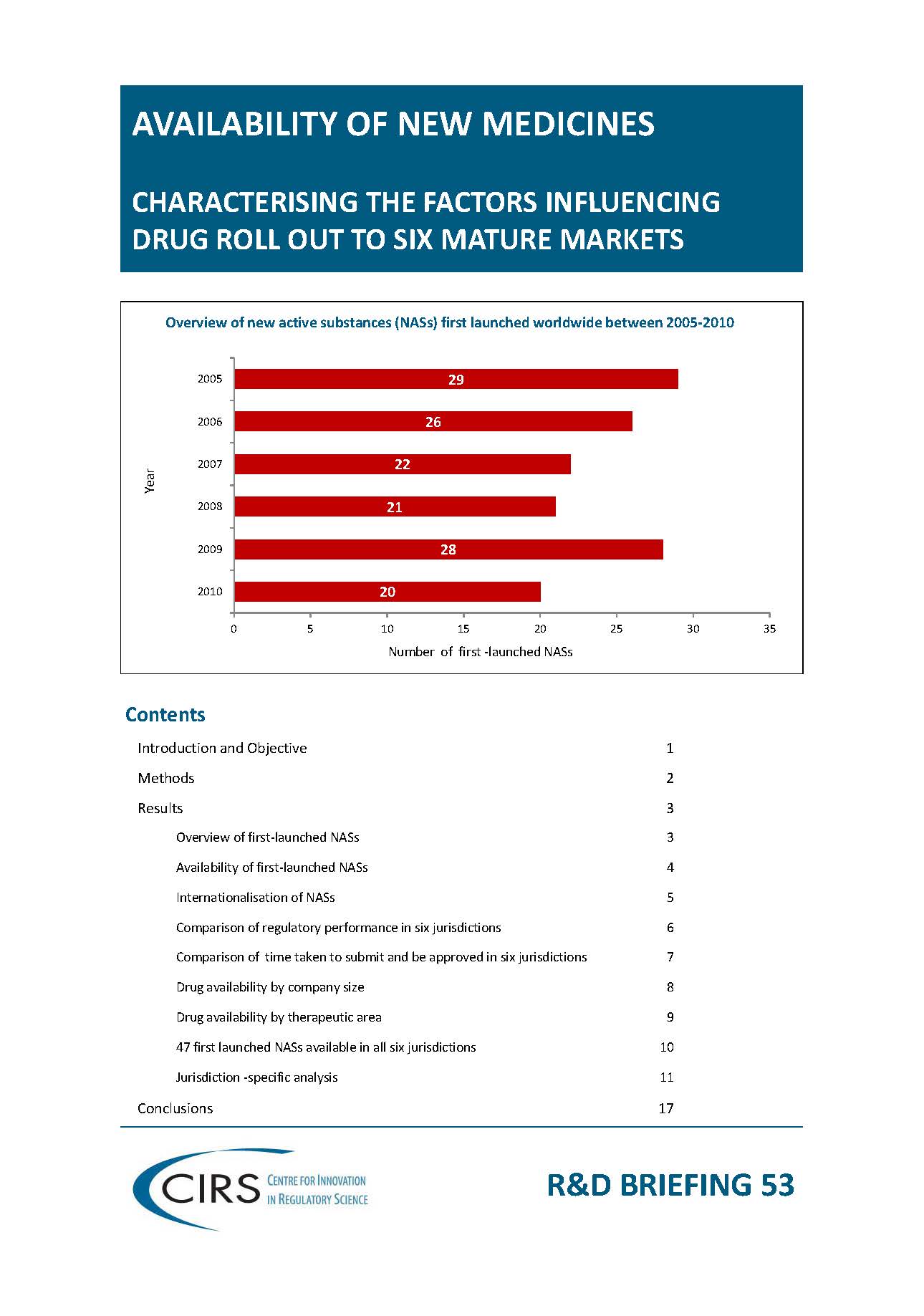
CIRS RD Briefing 53 – Factors influencing drug roll out to six mature markets
Objective: To review NASs first launched between 2005‐2010 and to determine their regulatory status as of 31 December 2012 in USA, Europe, Japan, Canada, Switzerland and Australia to identify [...]
CIRS RD Briefing 52 – New drug approvals in ICH countries 2003-2012
Active Substances (NASs) approved by both the FDA and PMDA represented the largest number of new medicines approved this decade. Regulatory approvals by EMA were lower than the other [...]