R&D Briefings
To keep up-to-date with the latest CIRS publications, request to be on our mailing list.
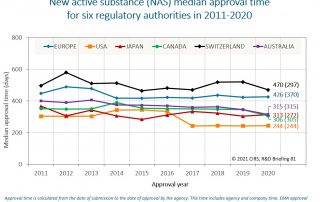
CIRS RD Briefing 81 – New drug approvals in six major authorities 2011-2020
This Briefing presents the results from the CIRS annual analysis of New Active Substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food [...]
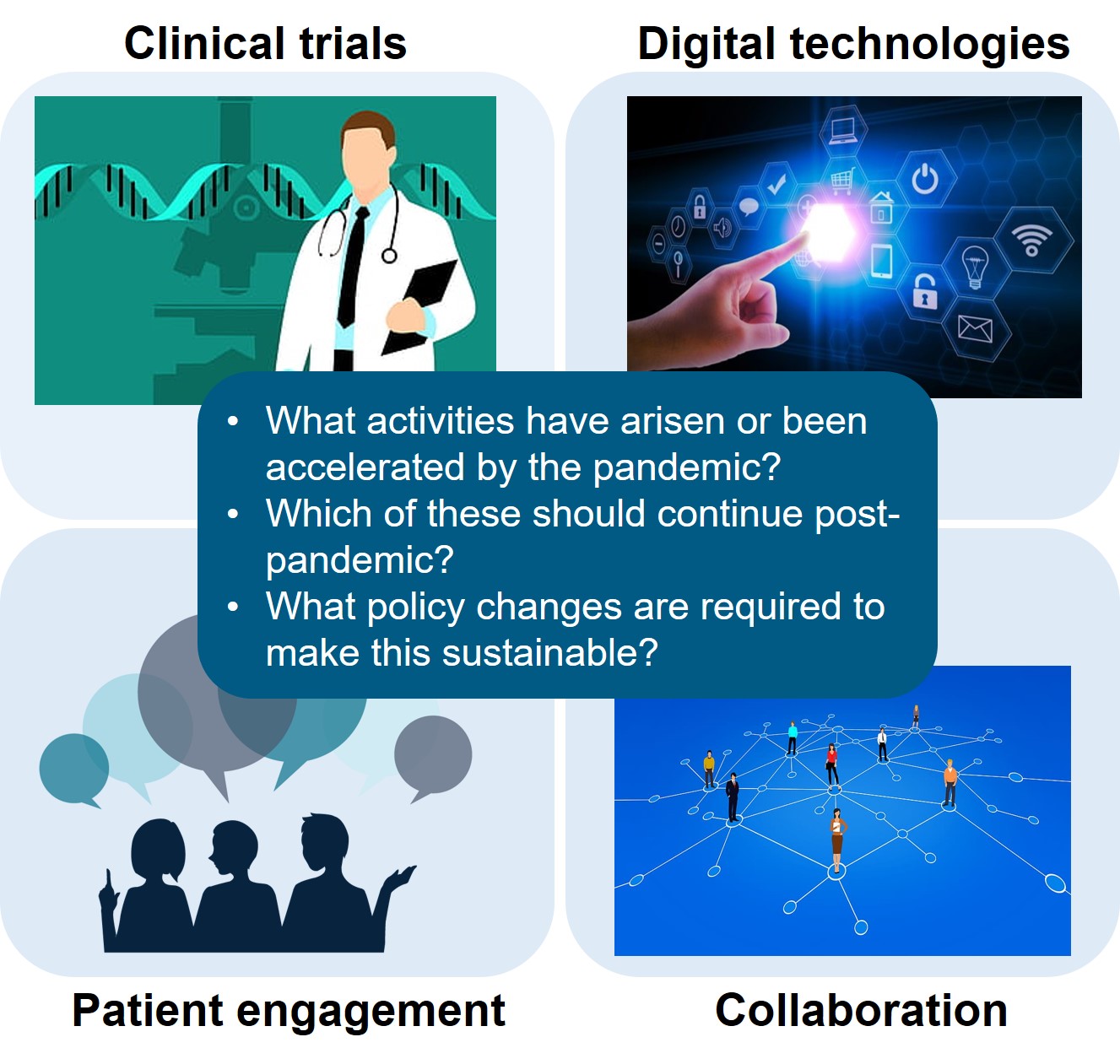
CIRS RD Briefing 80 – Reimagining medicine regulatory models
This R&D Briefing summarises the outputs of breakout group discussions held during a CIRS multi-stakeholder workshop in December 2020 entitled ‘Reimagining medicine regulatory models: implementing fit-for-purpose sustainable activities for [...]
CIRS RD Briefing 79 – Use of advisory committees in Colombia
This Briefing provides an overview of how advisory committees can be used to support the regulatory decision-making process and considering the context of Latin American regulatory systems, aims to [...]
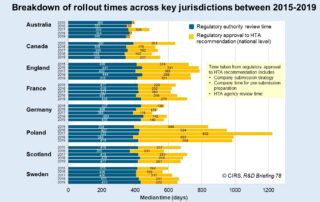
CIRS RD Briefing 78 – HTA outcomes in Australia, Canada and Europe 2015-2019
This Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory [...]
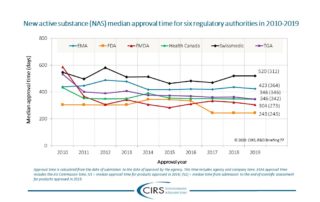
CIRS RD Briefing 77 – New drug approvals in six major authorities
This Briefing presents the results from the CIRS annual analysis of New Active Substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food [...]
CIRS RD Briefing 76 – Mexican therapeutic landscape
An efficient regulatory process can be reflected in measurable positive health impacts; conversely, activities that slow or impede regulatory efficiency and predictability can be detrimental. Recent developments in the [...]
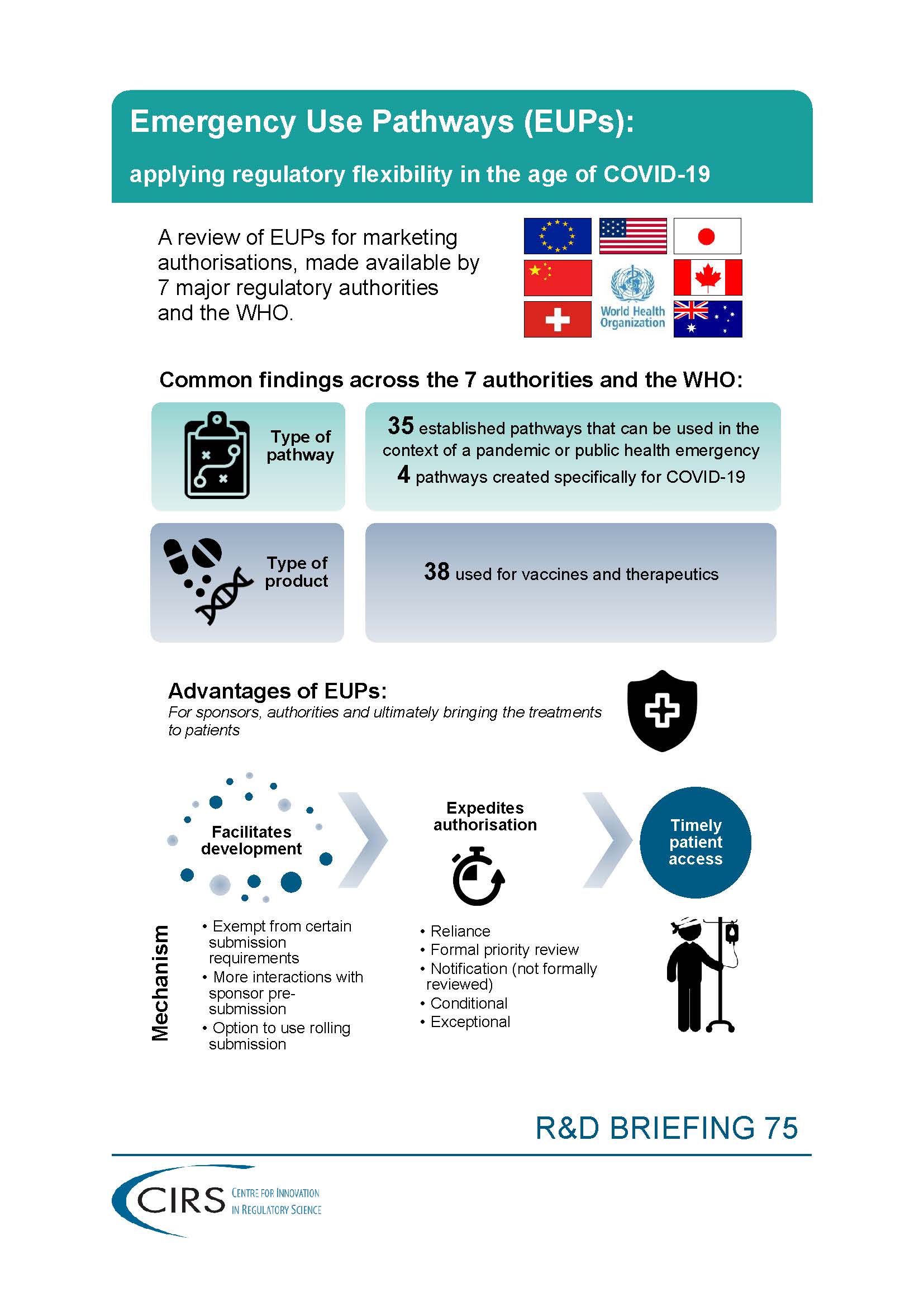
CIRS RD Briefing 75 – Emergency Use Pathways (EUPs)
It has become clear that agencies have a number of pathways that can be used during public health emergencies for the authorisation of therapeutics and vaccines. Some of these [...]
CIRS RD Briefing 74 – OpERA programme
CIRS has collected regulatory assessment data for over 20 years, initially with ICH and ICH-observing countries. The OpERA programme, “Optimising Efficiencies in Regulatory Agencies (OpERA)”, was initiated through CIRS [...]
CIRS RD Briefing 73 – HTA outcomes 2014-18
Timely recommendation for drug reimbursement by health technology assessment (HTA) agencies is critical to ensure that patient access to medicines of therapeutic value is not delayed. As part of an [...]
CIRS RD Briefing 71 – Trends in the regulatory landscape Latin America
To address the complex challenges in the global regulatory environment and the growing demand for patient access to new medicines, regulatory agencies in Latin America are actively engaging in regulatorystrengthening [...]