R&D Briefings
To keep up-to-date with the latest CIRS publications, request to be on our mailing list.
CIRS RD Briefing 102 – Tracking Availability in China of Medicines Approved in Six Key Global Markets
All approved medicines have been rigorously assessed by regulatory authorities to ensure their benefits outweigh the risks. Today, pharmaceutical companies are increasingly focused on integrated global drug development strategies, [...]
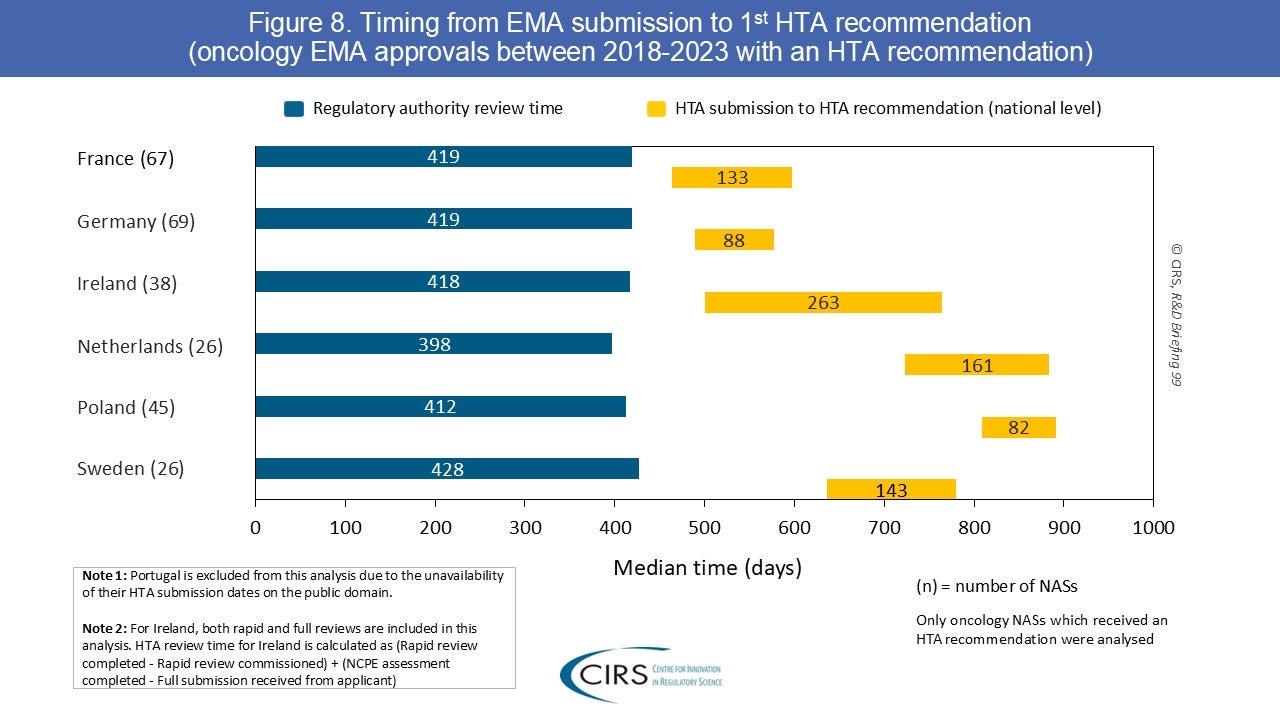
CIRS RD Briefing 99 – First HTA outcomes and timelines for oncology medicines approved by EMA 2018-2023
This R&D Briefing presents data from HTADock, an ongoing CIRS benchmarking study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies. This CIRS [...]
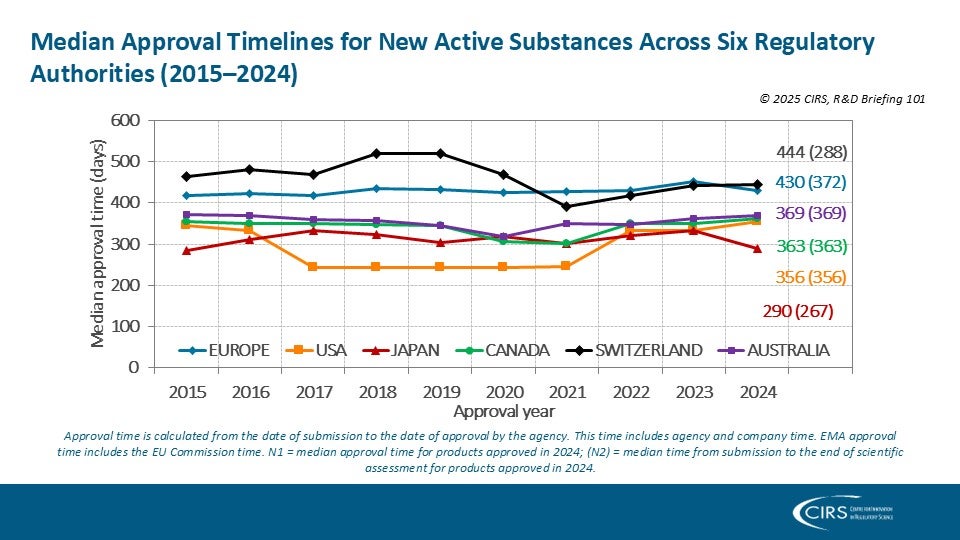
CIRS RD Briefing 101 – New drug approvals by six major authorities 2015-2024
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the [...]
CIRS RD Briefing 97 – Access Consortium and Project Orbis Approvals Across Eight Regulators
This R&D Briefing builds upon the Centre for Innovation in Regulatory Science (CIRS)'s long-standing efforts to examine trends and practices in regulatory approvals. For over 20 years, CIRS has [...]
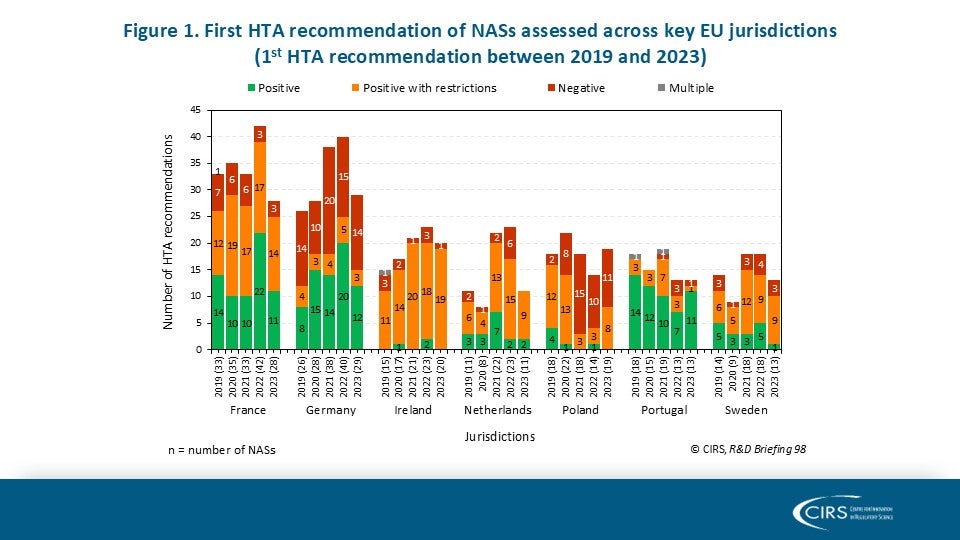
CIRS RD Briefing 98 – European HTA trends: HTA outcomes and timelines across seven markets 2019-2023
This R&D Briefing presents data from HTADock, an ongoing CIRS metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies. It [...]
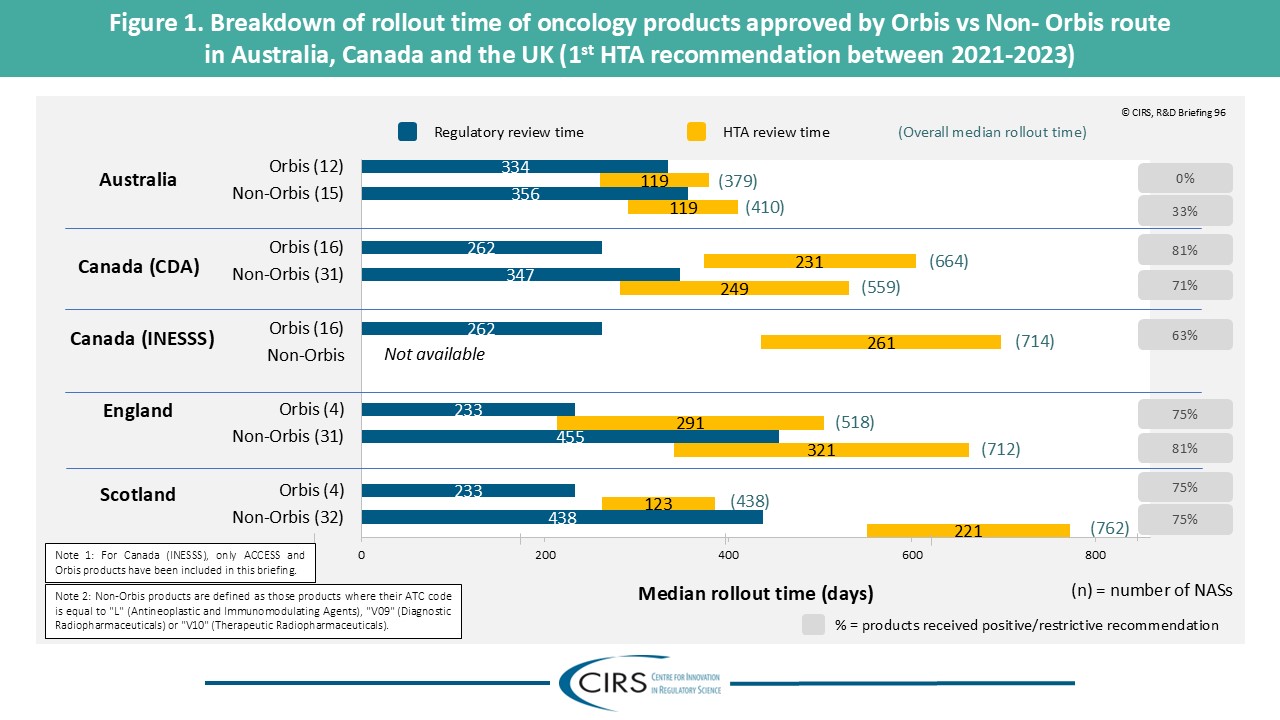
CIRS RD Briefing 96 – Review of HTA outcomes and timelines in Australia, Canada and the UK 2019-2023
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies. It focuses [...]
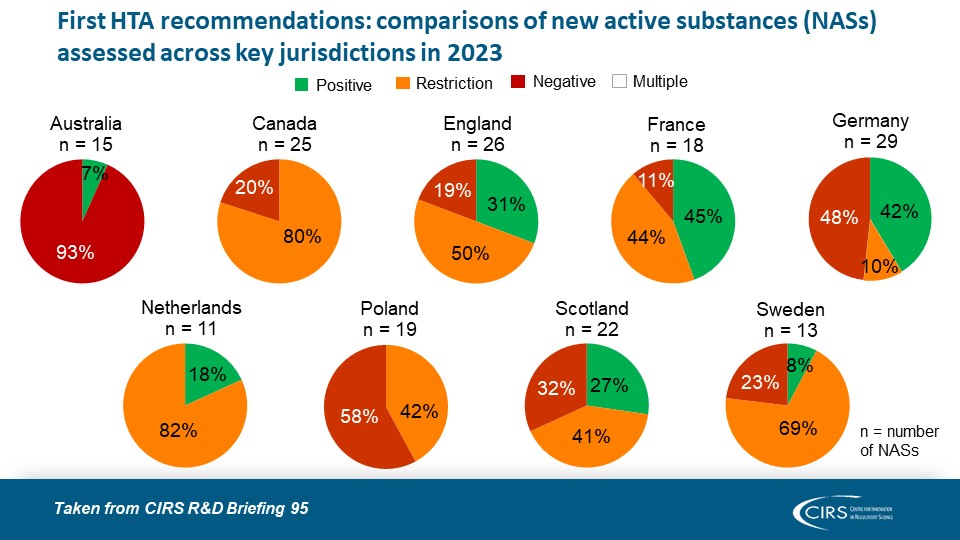
CIRS RD Briefing 95 – Review of HTA outcomes and timelines in Australia, Canada, Europe and the UK 2019-2023
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies, each with [...]
CIRS RD Briefing 93 – New drug approvals by six major authorities 2014-2023
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the [...]
CIRS RD Briefing 94 – Value of Reference Agency Reports in Enabling Reliance
Access to information, including the assessment documents of reference national regulatory agencies (NRA), is a key enabler of regulatory risk-based decision making. It promotes an understanding of what was [...]
CIRS RD Briefing 92 – Appraising the usability of public assessment reports for reliance
Regulatory reliance facilitates regulatory approvals, allows the use of resources more efficiently, and ultimately serves patients by accelerating access to quality-assured, safe, and effective medicines. The World Health Organisation [...]