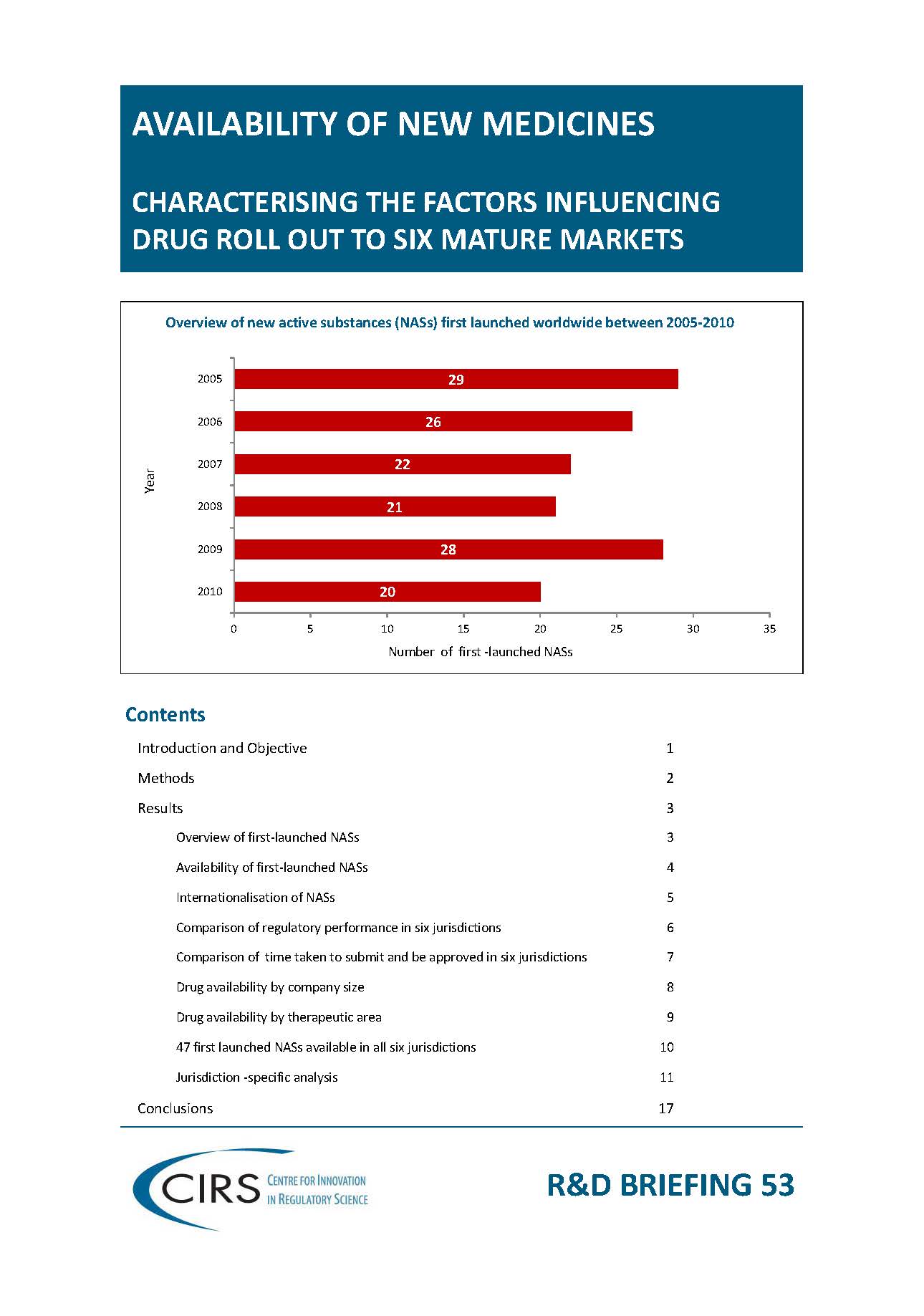
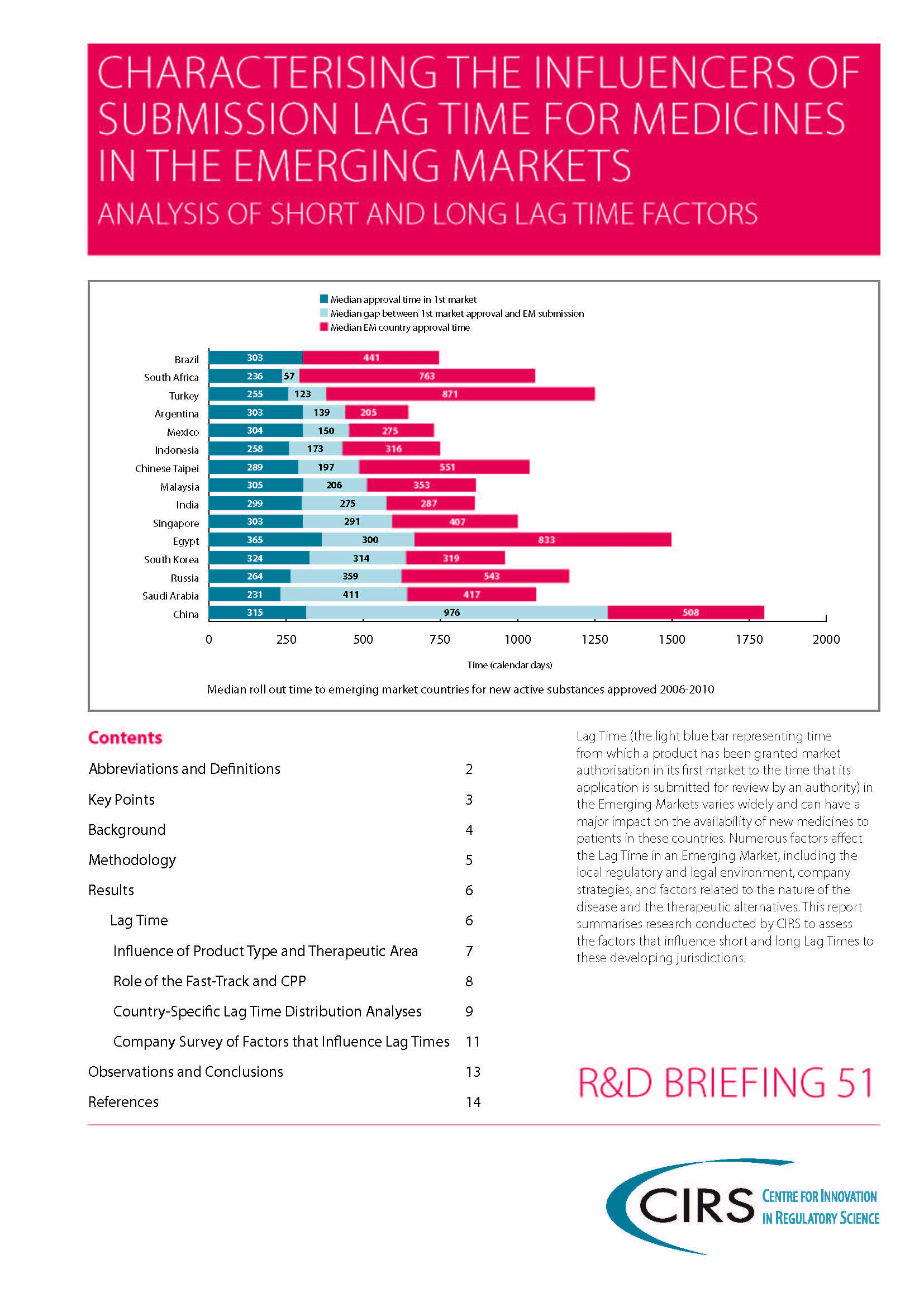
CIRS RD Briefing 53 – Factors influencing drug roll out to six mature markets
Objective: To review NASs first launched between 2005‐2010 and to determine their regulatory status as of 31 December 2012 in USA, Europe, Japan, Canada, Switzerland and Australia to identify [...]