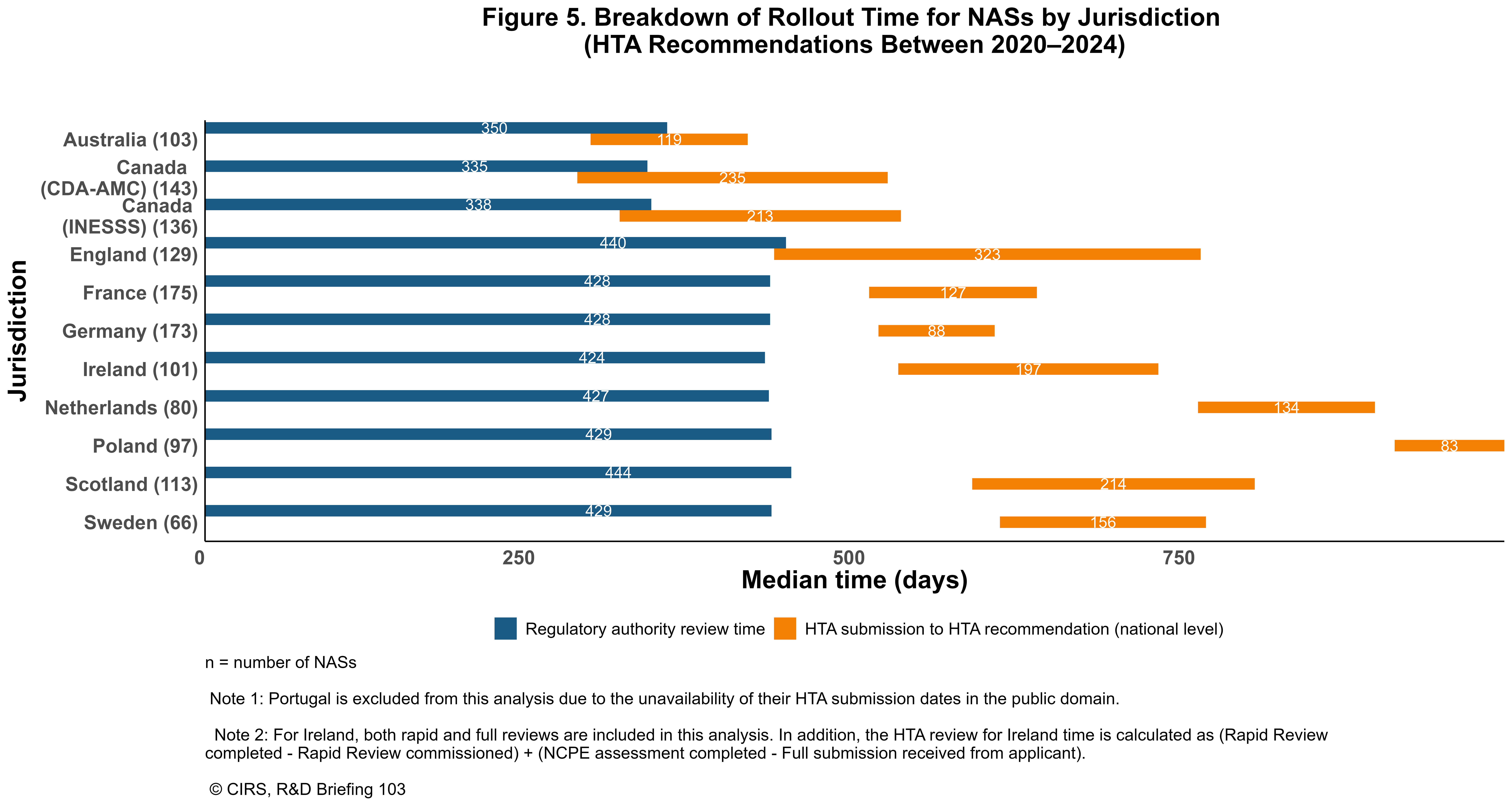
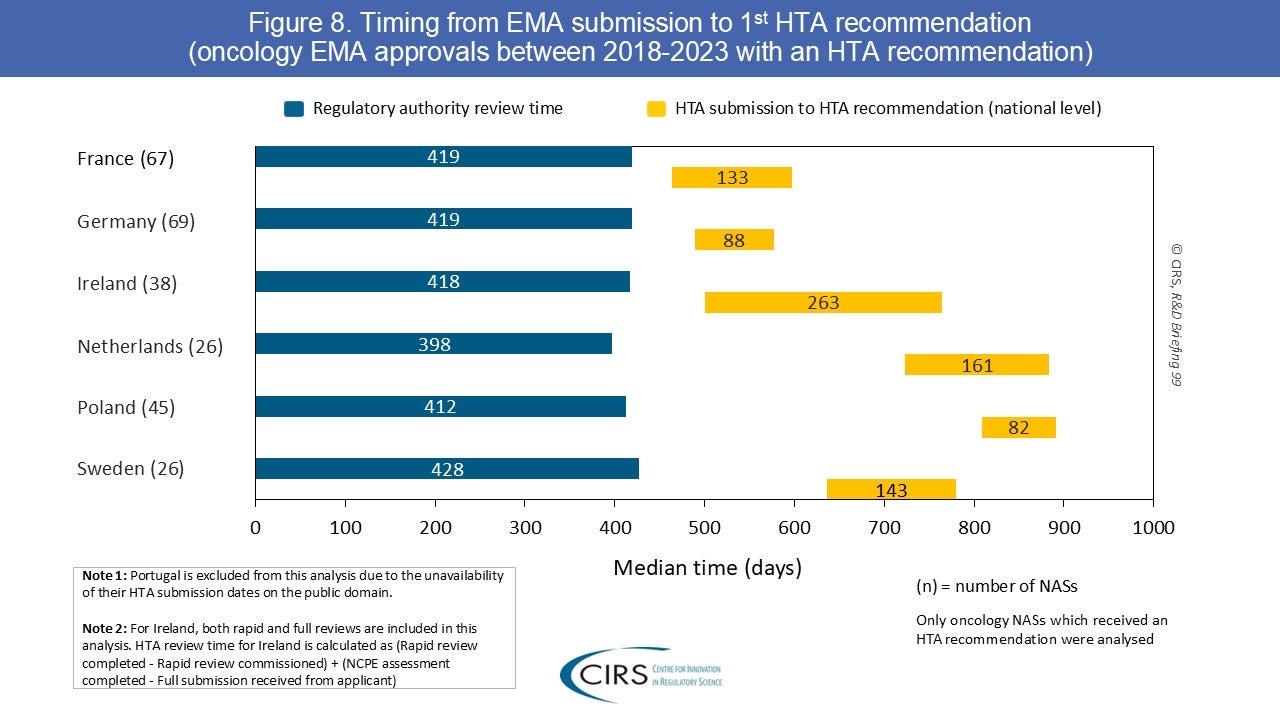
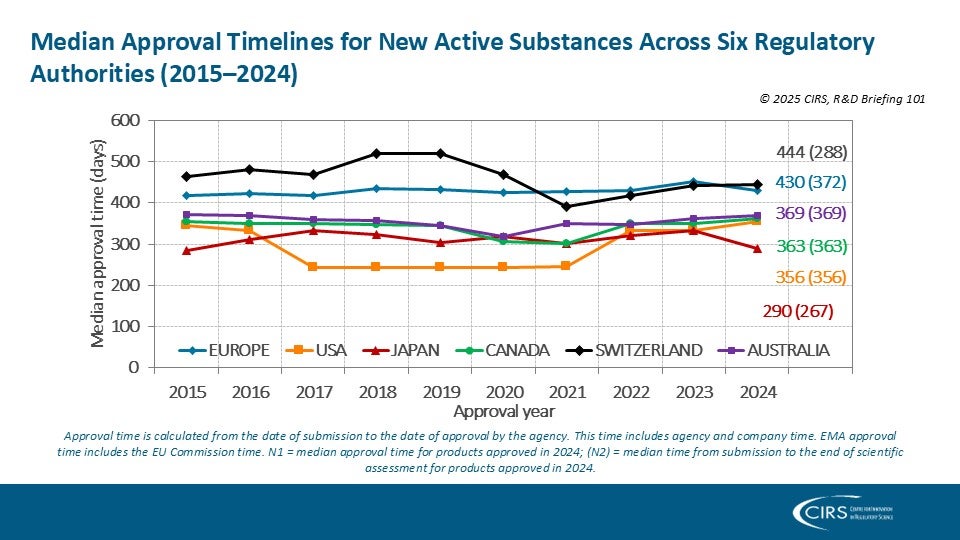
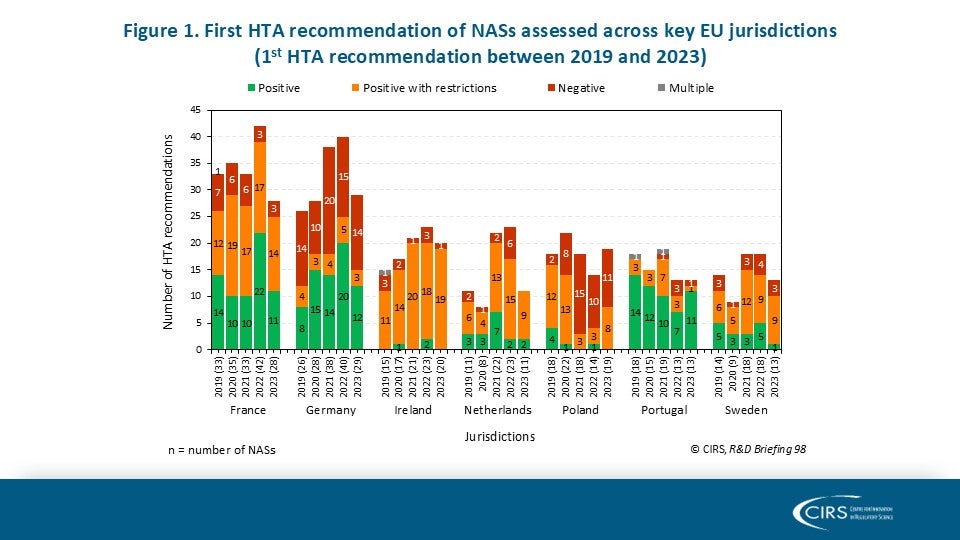
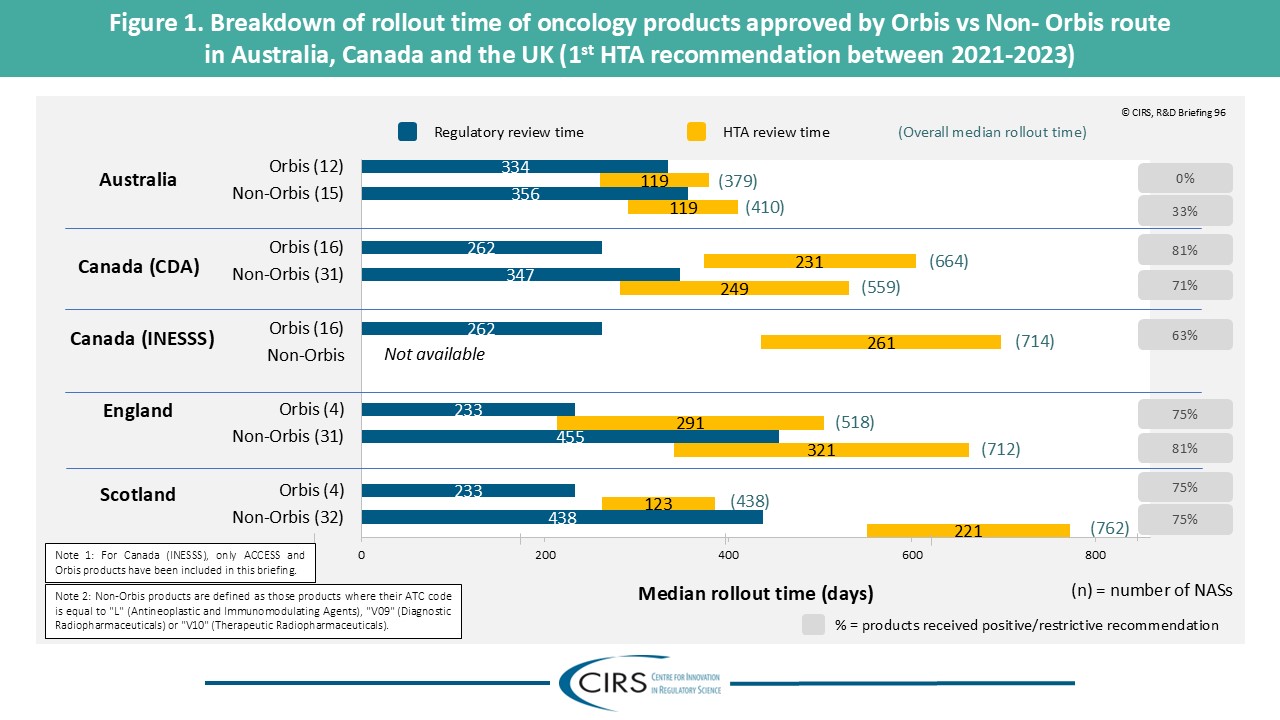
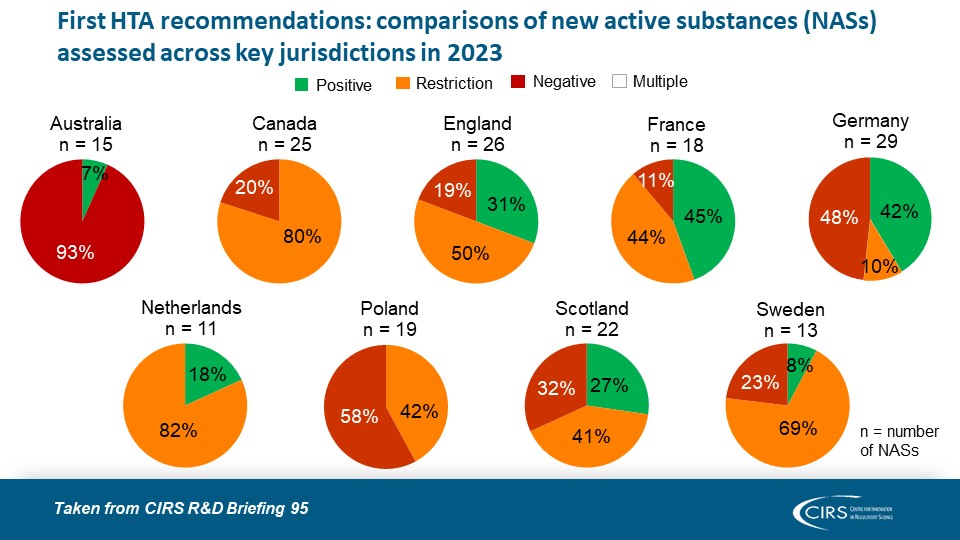
CIRS R&D Briefing 100: Four Decades of Evidence, Insight and Impact
Explore how our R&D Briefings have impacted regulatory and health technology assessment (HTA) policy worldwide. We are delighted to share with you CIRS R&D Briefing 100, a special edition [...]