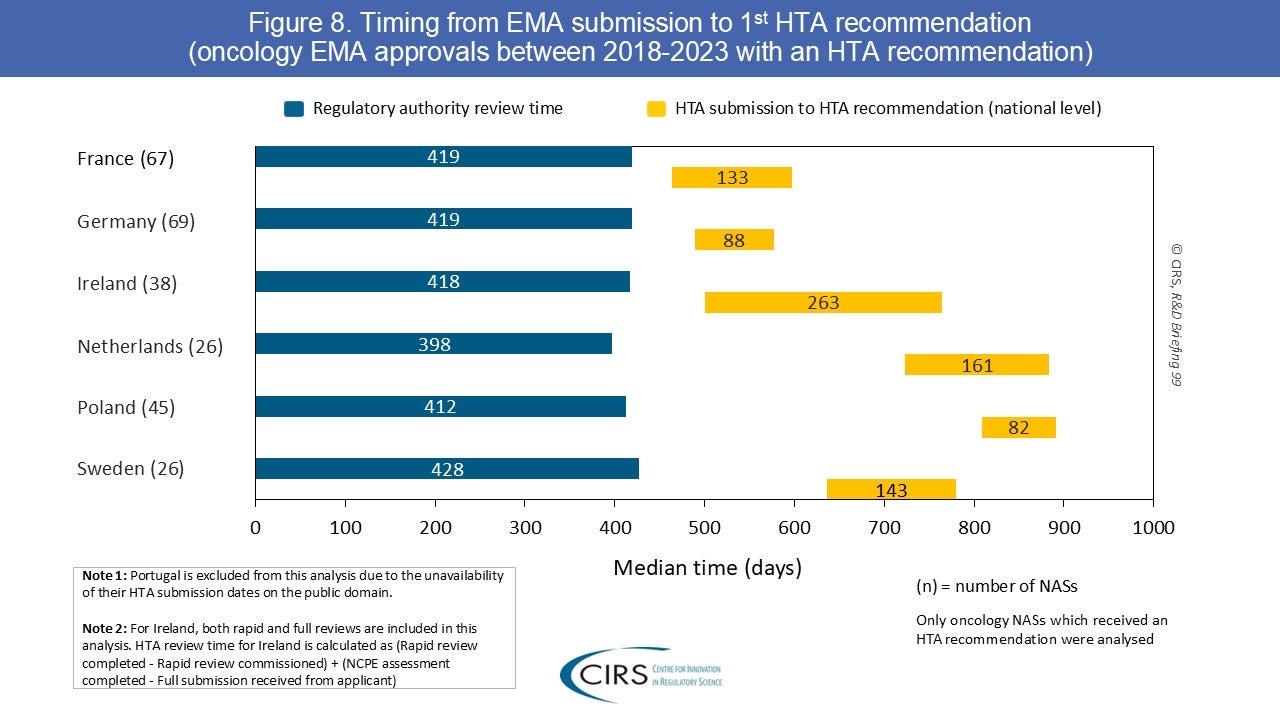
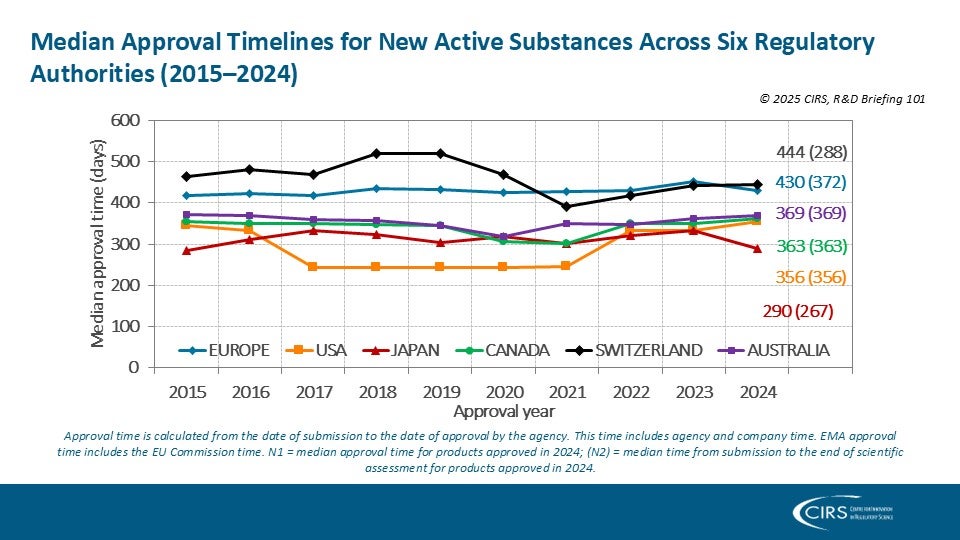
Assessing the Malaysian Regulatory Process with OpERA Methodology – Mohd Sani 2025
Background: The Malaysian National Pharmaceutical Regulatory Agency (NPRA) has partnered with the Centre for Innovation in Regulatory Science (CIRS) since 2018 to analyze the approval processes for new active [...]