Health technology assessment (HTA) is a multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its lifecycle (O’Rourke 2020). The purpose is to inform decision making in order to promote an equitable, efficient, and high-quality health system.
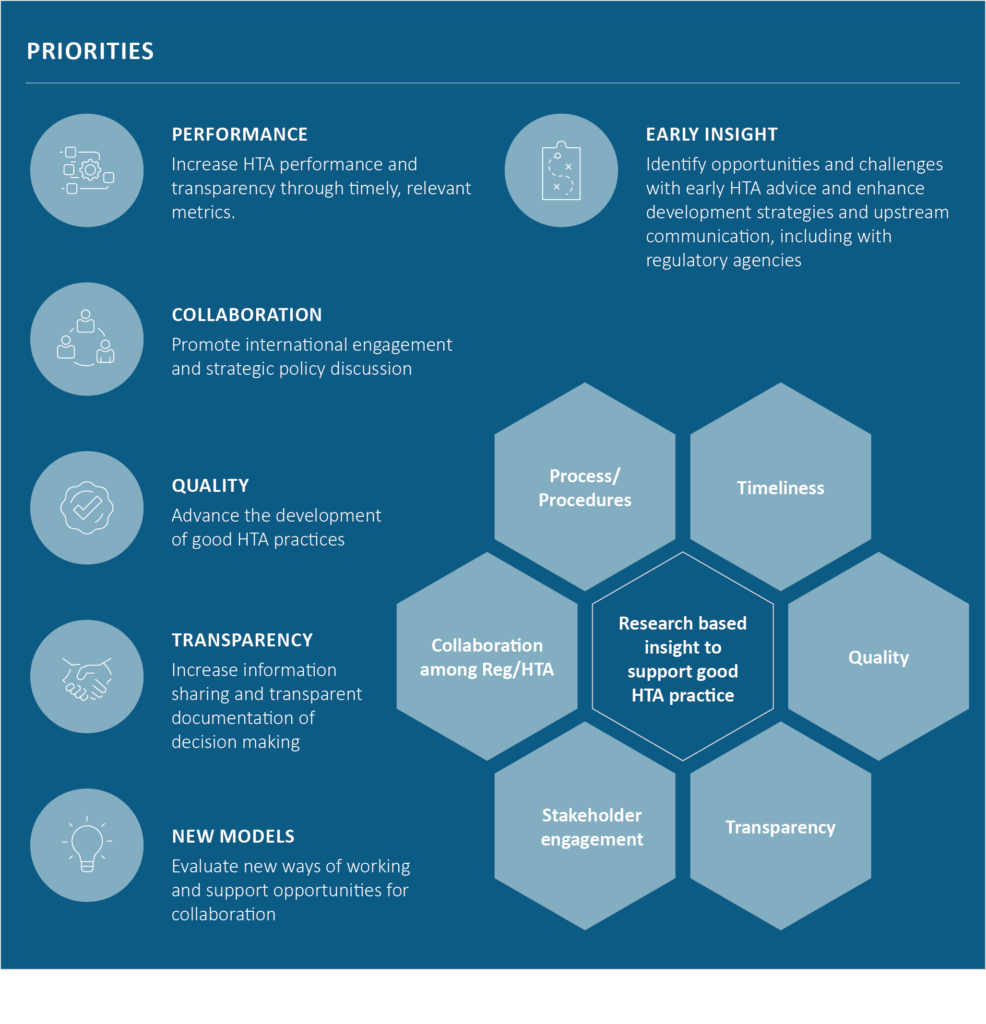
Over the past decade, HTA has evolved from a traditionally downstream decision focused on reimbursement to an increasingly upstream enabler shaping evidence generation and development strategy. Through its research and convening power, CIRS has tracked and supported this evolution, highlighting how HTA agencies are embedding themselves earlier in the development process, engaging in scientific advice, and collaborating more closely with regulatory counterparts.