How quickly do oncology medicines move from EMA approval to HTA submission across Europe — and how might this change under the EU HTA Regulation?
At ISPOR Europe 2025, we presented a poster sharing insights from a study examining HTA submission trends for EMA-approved oncology medicines across Europe between 2018-2023.
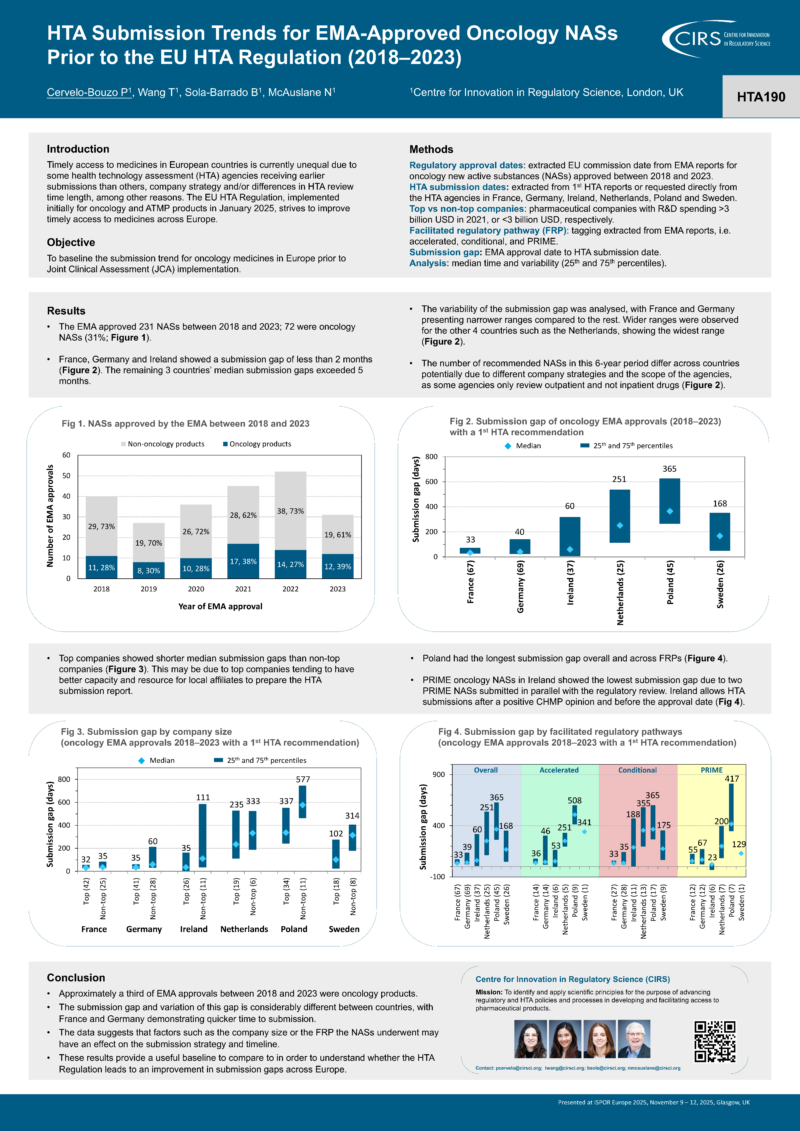
As oncology products are among the first therapies to undergo Joint Clinical Assessment (JCA), we focused exclusively on EMA oncology approvals and their corresponding national HTA decisions — providing a baseline to support comparison and learning as JCA is implemented.
Key insights from our analysis:
- Approximately a third of EMA approvals between 2018 and 2023 were oncology products.
- Time to HTA submission varies considerably across countries, with France and Germany showing faster submission times.
- Company size and regulatory pathways appear to influence submission strategy and timing.
- These findings offer an important reference point to assess whether the EU HTA Regulation reduces submission gaps over time.
This poster was presented by Penelope Cervelo at ISPOR Europe 2025, 9-12th November 2025, Glasgow, UK.
Conference abstract
Objectives: Access to medicines in European countries is currently unequal, with some HTA agencies receiving earlier submissions than others. The HTA Regulation, implemented for oncology products in January 2025, strives to improve timely access to medicines across Europe. This study aims to baseline the submission trend to oncology medicines in Europe prior to JCA implementation.
Methods: The regulatory approval dates were extracted from EMA reports from oncology NASs approved between 01-Jan-2018 to 31-Dec-2023. The HTA submission dates were extracted or requested for France, Germany, Ireland, Netherlands, Poland and Sweden. The submission gap is defined as EMA approval date to HTA submission date, median time and variability with 25th and 75th percentiles were calculated.
Results: The EMA approved 231 NASs between 2018 and 2023, 72 were oncology NASs (31%). Three countries showed a submission gap of less than 2 months, France, Germany and Ireland, with 33, 40 and 60 days respectively. While the remaining 3 countries’ median submission gaps exceeded 5 months. The variability of the submission gap was also analysed, and France and Germany presented narrower ranges (29 to 69 days, 26 to 138 days, respectively) compared to the rest. Wider ranges were observed in the other 4 countries such as Netherlands, showing the widest range from 116 to 535 days. Factors that may affect submission strategy such as company size, conditional and accelerated EMA approvals were also explored.
Conclusions: Our analysis demonstrated that a high proportion of EMA approvals between 2018 and 2023 were oncology products. The submission gap and variation of this gap is considerably different between countries, with France and Germany demonstrating quicker time to submission. These results will provide a useful baseline to compare if the regulation leads to an improvement in submission gaps across Europe.