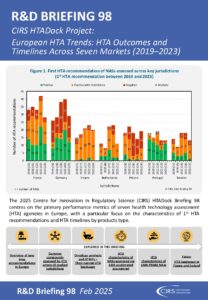
This R&D Briefing presents data from HTADock, an ongoing CIRS metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies.
It focuses on performance metrics of seven HTA agencies in Europe:
- French Haute Autorité de Santé (HAS)
- German Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG)
- Irish National Centre for Pharmacoeconomics (NCPE)
- Zorginstituut Nederland (ZIN)
- Polish Agencja Oceny Technologii Medycznych i Taryfikacji (AOTMiT)
- Portuguese Autoridade Nacional do Medicamento e Produtos de Saude (INFARMED)
- Swedish Tandvårds & läkemedelsförmånsverket (TLV).
Our analyses focus on 1st HTA recommendations and rollout timelines, including for oncology products, ATMPs, products using EMA accelerated assessment and PRIME priority medicines. We also assessed the impact of Early Access and Rapid Review mechanisms in France and Ireland, respectively.
These insights act as an important baseline for measuring the impact of the EU HTA Regulation, which is now in play for oncology products and ATMPs, and will gradually be rolled out to cover all centrally authorised products by 2030.
If you have any questions or comments on this briefing, please get in touch with Dr Tina Wang: twang@cirsci.org