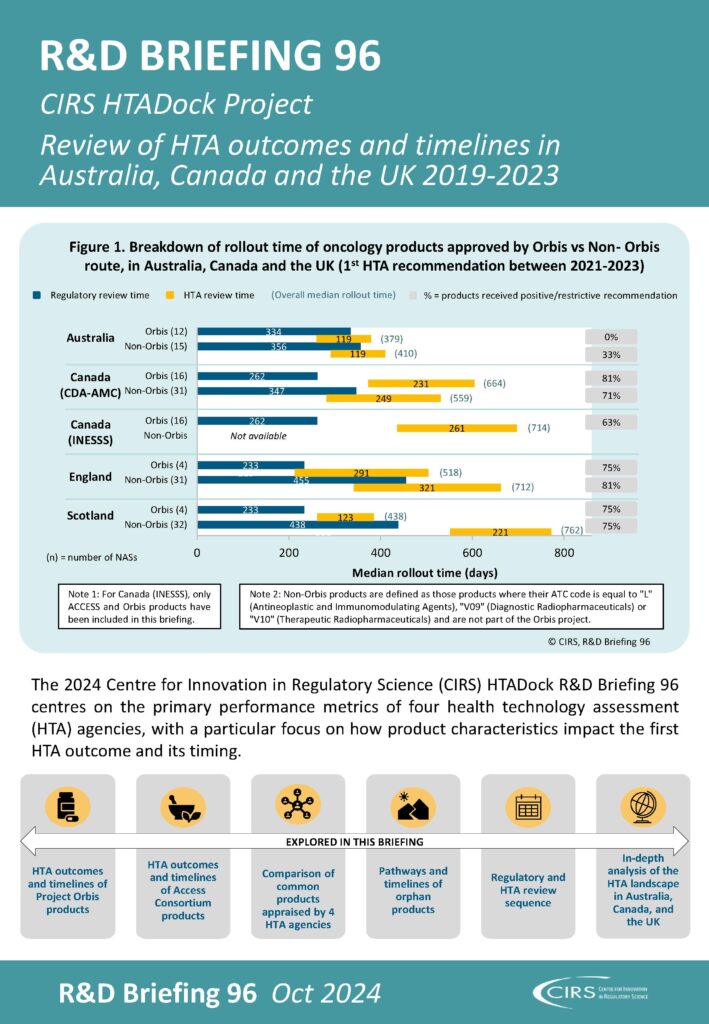
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies.
It focuses on performance metrics of four HTA agencies in Australia, Canada and the UK (listed below*), including regulatory and HTA review sequence, common products appraised by all four agencies, as well as the HTA outcome and timing for products approved via the Access Consortium and Project Orbis*.
- Australian Pharmaceutical Benefits Advisory Committee (PBAC)
- Canadian Drug Agency (CDA-AMC)
- English National Institute for Health and Care Excellence (NICE)
- Scottish Medicines Consortium (SMC).
If you have any questions or comments on this Briefing, please don’t hesitate to get in touch with Dr Tina Wang: twang@cirsci.org
*The Institut national d’excellence en santé et en services sociaux (INESSS) was also included in this briefing for analyses of the Access Consortium and Project Orbis. The Medical Services Advisory Committee (MSAC) was included for an analysis on TGA priority review.