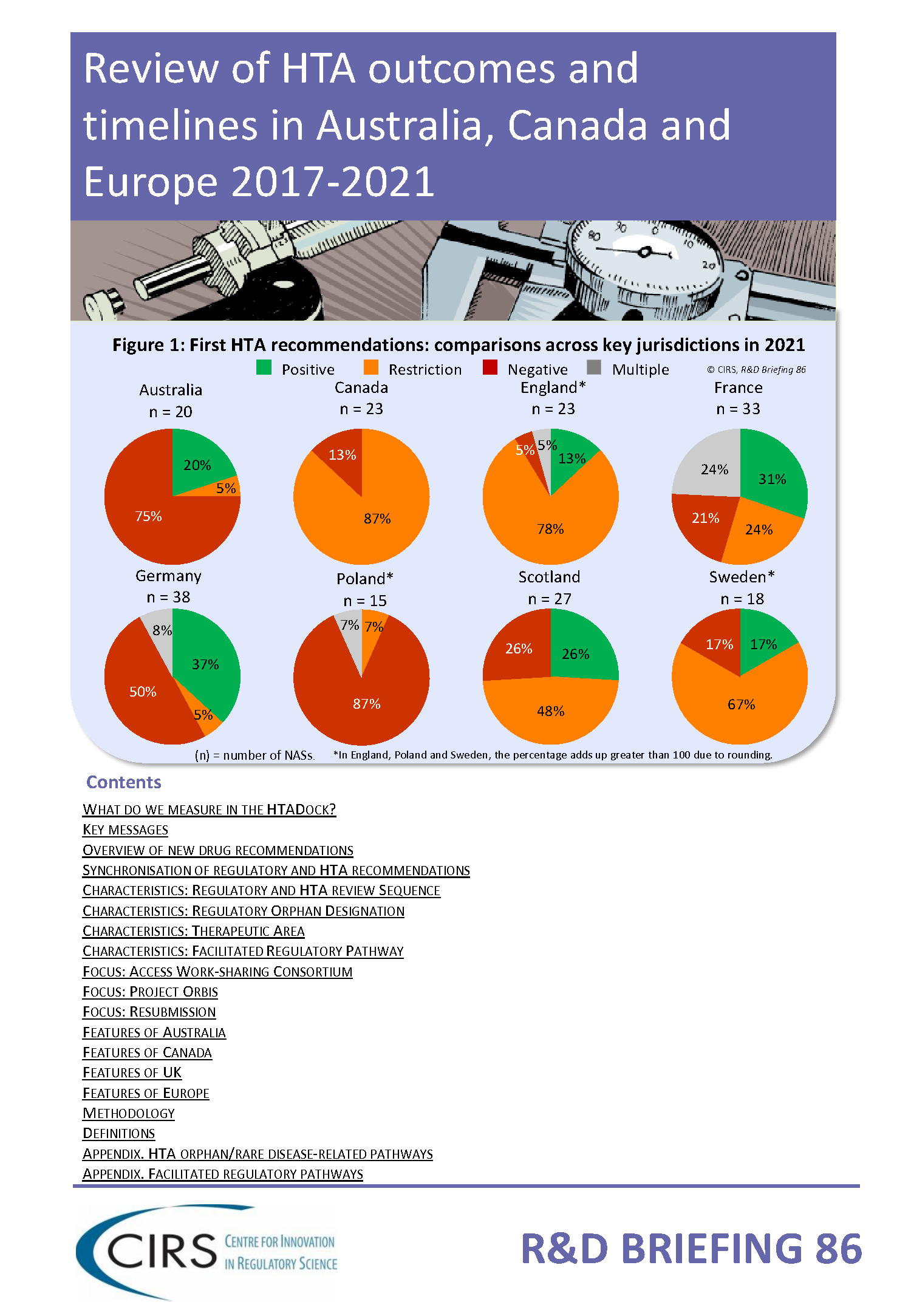
The Briefing presents data from HTADock, an ongoing metrics study that collects data on new active substances (NASs) appraised by eight HTA agencies and analyses synchronisation between the regulatory decision and first HTA recommendation in terms of timing and outcome. This year we have also analysed the impact of regulatory review type, the resubmission status of HTA assessment, and explored regulatory and HTA decision-making for products that underwent Project Orbis and Access Consortium.
Recommendations were collected from the Australian Pharmaceutical Benefits Advisory Committee (PBAC), Canadian Agency for Drugs and Technologies in Health (CADTH; both Common Drug Review [CDR] and pan-Canadian Oncology Drug Review [pCODR]), English National Institute for Health and Care Excellence (NICE), French Haute Autorité de Santé (HAS), German Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG), Polish Agencja Oceny Technologii Medycznych i Taryfikacji (AOTMiT), Scottish Medicines Consortium (SMC) and Swedish Tandvårds- & läkemedelsförmånsverket (TLV), for NASs approved between 2017-2021 by the respective jurisdictional regulatory agencies, the Australian Therapeutic Goods Administration (TGA), Health Canada and European Medicines Association (EMA).
If you have any questions or comments on this Briefing, please don’t hesitate to get in touch with Tina Wang: twang@cirsci.org