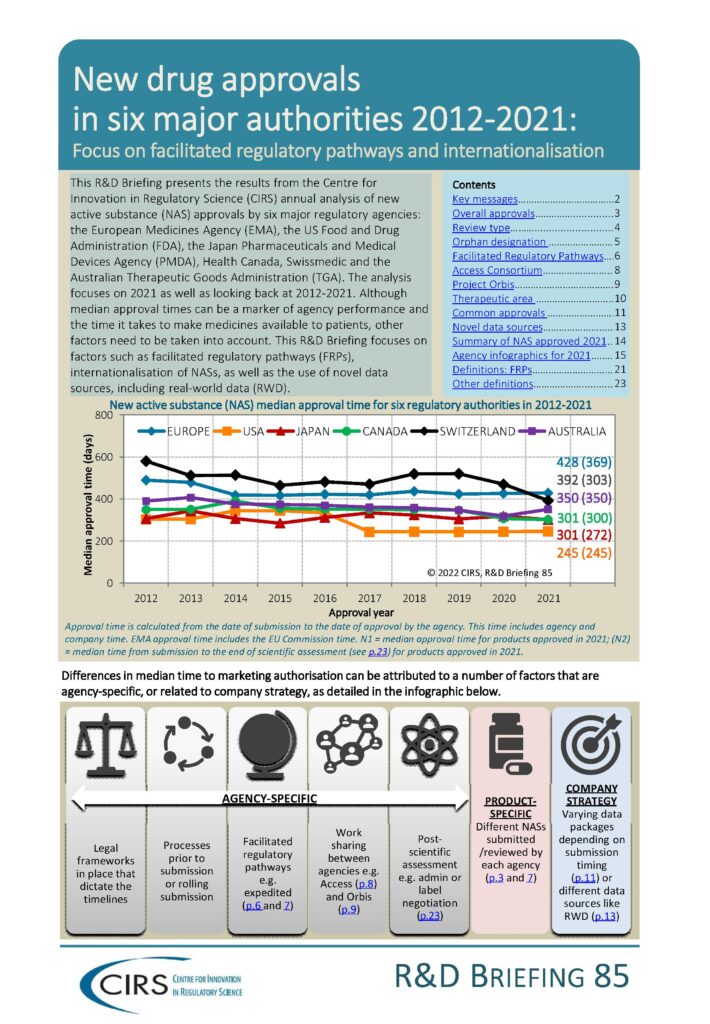
This Briefing presents the results from the CIRS annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food and Drug Administration (FDA), the Japan Pharmaceuticals and Medical Devices Agency (PMDA), Health Canada, Swissmedic and the Australian Therapeutic Goods Administration (TGA). The analysis focuses on 2021 as well as looking back at 2012-2021.
Although median approval times can be a marker of agency performance and the time it takes to make medicines available to patients, other factors need to be taken into account. This R&D Briefing focuses on factors such as facilitated regulatory pathways, internationalisation of NASs, as well as the use of novel data sources, including real-world data.
The NAS list associated with this R&D Briefing is available to download here.
If you have any questions or comments on this R&D Briefing, please don’t hesitate to get in touch with Magda Bujar: mbujar@cirsci.org