Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
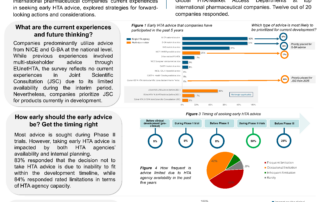
Navigating HTA Requirements During Development Through Early HTA Scientific Advice
Background Pharmaceutical companies have been actively taking early scientific advice from health technology assessment (HTA) agencies during development, with the aim to understand the HTA evidentiary requirements. The evolving advice …
Comparison of Three Regional Medicines Regulatory Harmonisation Initiatives in Africa
Background The African Medicines Regulatory Harmonisation (AMRH) Initiative was formed in 2009 and subsequently, three regional initiatives (East African Community Medicines Regulatory Harmonisation [MRH], Southern African Development Community [SADC]/ZaZiBoNa MRH, …
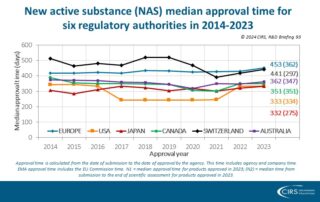
CIRS RD Briefing 93 – New drug approvals by six major authorities 2014-2023
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the European …
Assessing the use of risk-based approaches in four major agencies
Introduction Over the last years, several regulatory agencies have developed risk-based approaches for the regulatory assessment of marketing authorisations of New Active Substances (NASs) as strategies to efficiently use limited …
CIRS 2023 Annual Report
We’re delighted to present our latest Annual Report, which provides a summary of the projects, publications, multi-stakeholder workshops and Technical Fora from Regulatory and HTA workstreams undertaken in 2023. The …
CIRS RD Briefing 94 – Value of Reference Agency Reports in Enabling Reliance
Access to information, including the assessment documents of reference national regulatory agencies (NRA), is a key enabler of regulatory risk-based decision making. It promotes an understanding of what was reviewed …
2024 Workshop Synopsis – What is needed for risk-based approaches to work effectively and efficiently?
In this workshop, CIRS brought together senior representatives from regulatory agencies, pharmaceutical companies and academia from 18 countries across the Americas, Africa, Asia and Europe, to examine risk-based approaches in …
CIRS RD Briefing 92 – Appraising the usability of public assessment reports for reliance
Regulatory reliance facilitates regulatory approvals, allows the use of resources more efficiently, and ultimately serves patients by accelerating access to quality-assured, safe, and effective medicines. The World Health Organisation (WHO) …
2023 Workshop report – Review and reimbursement frameworks for rare disease products
There are an estimated 300 million people across the world affected by around 7000 known rare diseases. Challenges in bringing treatments to market for these conditions include small patient populations, …
2023 Workshop report – Uncertainty in the development of new medicines
This CIRS workshop brought together companies and agencies (HTA and Regulatory) to discuss the sources of uncertainty that are being built in, by the way medicines development has evolved and …