Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
Regulatory Collaboration and System Strengthening – Workshop Report
This CIRS multi-stakeholder workshop examined success factors for strengthening regulatory systems to support the implementation of collaborative models.
Assessing the Malaysian Regulatory Process with OpERA Methodology – Mohd Sani 2025
Background: The Malaysian National Pharmaceutical Regulatory Agency (NPRA) has partnered with the Centre for Innovation in Regulatory Science (CIRS) since 2018 to analyze the approval processes for new active substances …
CIRS 2024 Annual Report
Explore how the Centre for Innovation in Regulatory Science (CIRS) is shaping the future of regulatory and health technology assessment (HTA) policy worldwide. We’re delighted to present our latest Annual …
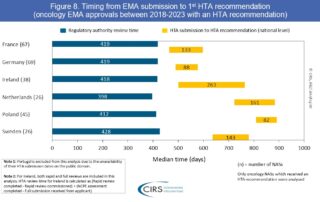
CIRS RD Briefing 99 – First HTA outcomes and timelines for oncology medicines approved by EMA 2018-2023
This R&D Briefing presents data from HTADock, an ongoing CIRS benchmarking study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies. This CIRS R&D …
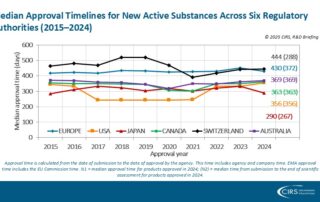
CIRS RD Briefing 101 – New drug approvals by six major authorities 2015-2024
This R&D Briefing presents the results from the Centre for Innovation in Regulatory Science (CIRS) annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the European …
Comparison of review models and timelines in ECOWAS – Owusu-Asante 2025
Introduction National regulatory medicines authorities (NRAs) are mandated to ensure timely access to high-quality, safe and efficacious medical products, primarily achieved through a marketing authorisation procedure established in each country. …
Structured benefit risk assessment: South African regulator case study – Danks 2025
This study investigates the utility of the Universal Methodology for Benefit-Risk Assessment (UMBRA) framework within the South African Health Products Regulatory Authority (SAHPRA) to determine whether adopting a structured approach …
Testing the UMBRA benefit-risk tool in a South African agency
Objectives Regulators must balance medicine benefits and risks while ensuring consistency, transparency, and efficiency in decision-making and the aim of this study was to assess UMBRA’s impact on SAHPRA to …
Assessing Good Review Practices at the FDA Ghana
Objectives To identify the current perspective of the FDA Ghana in the implementation of Good Review Practices, provide a baseline on the knowledge, attitudes, practices, as well as identify areas …
Proposed Model for EAC MRH Joint Review
Objectives 1. To investigate ways in which the regional initiative could be well coordinated. 2. To propose an improved model for the East African Medicine Regulatory Harmonisation Initiative joint assessments. …
Evaluating Quality of Decision Making within the Zambia Medicines Regulatory Authority
Objectives To assess the quality of the decision-making process followed by regulatory reviewers, explore their perceptions of the Zambia Medicines Regulatory Authority (ZAMRA) decision-making approach, and evaluate the usefulness of …
Regulatory Collaboration and System Strengthening – Workshop Synopsis
This CIRS multi-stakeholder workshop examined success factors for strengthening regulatory systems to support the implementation of collaborative models.
Workshop Report – Regulatory and HTA collaborative models
In this workshop, CIRS brought together senior representatives from regulators, HTA agencies, pharmaceutical companies, payers, academics and patient organisations to discuss the impact of regulatory and HTA collaborative models and …
Ensuring efficiency and effectiveness of Joint Clinical Assessment (JCA) – Wang 2025
Background: This study explored the readiness and strategic considerations of companies and key stakeholders for the implementation of the Joint Clinical Assessment (JCA) under the European Health Technology Assessment Regulation …