Publications
CIRS publishes insights from its research and meetings in several forms:
- R&D Briefings – research papers produced by the CIRS team e.g. annual regulatory and HTA benchmarking briefings
- Journal articles – peer reviewed academic research papers
- Reports – from CIRS workshops and externally commissioned research projects, as well as CIRS Annual Reports
- Books – research theses from CIRS-supported PhD students
- Posters – presented at external conferences
Keep up-to-date with CIRS publications and activities by signing up to our mailing list or following CIRS on LinkedIn.
CIRS RD Briefing 55 – Approvals across six major authorities 2004-2013
As part of the ongoing study to monitor regulatory performance, CIRS has analysed the trends in new medicines’ approval between 2004 and 2013 by six regulatory authorities including Health Canada, …
2013 Workshop report – Commonality across decision frameworks used by HTA and regulatory agencies
IS THERE A COMMONALITY ACROSS THE STRUCTURED DECISION FRAMEWORKS USED BY HTA AND REGULATORY AGENCIES? 1st-2nd October 2013, Surrey, UK This Workshop was designed to bring together the various stakeholders …
2013 Workshop report – Building quality into HTA/coverage decision-making processes
Building quality into HTA/coverage decision-making processes: What are the features of good practice in HTA? 2-3 December 2013, Heathrow, UK This workshop continued the work of the CIRS September 2011 …
2014 Workshop report – Focus on Latin America: Building quality submission and review processes and practices
Focus on Latin America: Building quality submission and review processes and practices – Overcoming challenges and meeting expectations 23-24 January 2014, Lima, Peru This workshop was held to discuss how …
2013 Workshop report – Implementing an international framework for benefit risk assessment
Implementing an internationally accepted framework for the benefit-risk assessment of medicines: How close are we to this objective? 20-21 June 2013, Washington DC, USA At the annual CIRS Benefit-Risk Workshop …
CIRS RD Briefing 54 – Approvals in ICH countries 2004-2013
In 2013, the overall number of New Active Substances (NASs) approved by EMA, FDA and PMDA was comparable across the three agencies. Nevertheless, despite this similarity, the number of NASs …
CIRS RD Briefing 53 – Factors influencing drug roll out to six mature markets
Objective: To review NASs first launched between 2005‐2010 and to determine their regulatory status as of 31 December 2012 in USA, Europe, Japan, Canada, Switzerland and Australia to identify the …
Liu et al 2013 – Characterising Good Review Practices across APEC agencies
As a first step in the implementation of the Asia-Pacific Economic Cooperation (APEC) Best Regulatory Practice Project, the Centre for Innovation in Regulatory Science conducted a gap analysis survey among …
2013 Workshop report – Patient voice in clinical development
Patient voice in clinical development: Can patients contribute to the benefit-risk assessment of new medicines? 13-14 March 2013, Surrey, UK The consensus of the April 2012 CIRS Workshop, The Patient’s …
2013 Workshop report – Ensuring quality of review decisions
Regulatory review: How do agencies ensure the quality of the decision? The role of decision frameworks in the review of new medicines: What are the challenges and solutions that can …
Allen et al 2013 – Archetypes for non-ranking classification and comparison of European HTA systems
Introduction: European countries are increasingly utilising health technology assessment (HTA) to inform reimbursement decision-making. However, the current European HTA environment is very diverse, and projects are already underway to initiate …
CIRS RD Briefing 52 – New drug approvals in ICH countries 2003-2012
Active Substances (NASs) approved by both the FDA and PMDA represented the largest number of new medicines approved this decade. Regulatory approvals by EMA were lower than the other agencies …
2012 Workshop report – Building the benefit-risk toolbox
Building the benefit-risk toolbox: Are there enough common elements across the different methodologies to enable a consensus on a scientifically acceptable framework for making benefit-risk decisions? 20-21 June 2012, Washington …
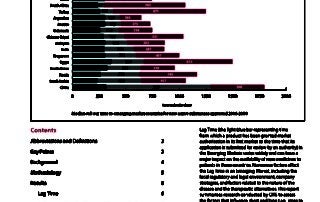
CIRS RD Briefing 51 – Submission lag time in the emerging markets
Lag Time (the time from which a product has been granted market authorisation in its first market to the time that its application is submitted for review by an authority) …
2012 Workshop report – Patient’s role in benefit-risk assessment
The patient’s role in the benefit-risk assessment for the submission and review of new medicines 25-26 April 2012, Hampshire, UK This workshop gained a perspective from various stakeholders in the …