More collaborative initiatives are taking place among regulatory agencies, health technology assessment (HTA) bodies and between regulatory and HTA agencies at national and regional levels. This blog by Martina Garau (Office of Health Economics) and Dr. Tina Wang (CIRS) provides a framework to navigate key aims, challenges and opportunities offered by recent initiatives and reflects on future prospects.
Healthcare decision makers face increasing pressure to review many new and complex interventions with robust methods, fast timelines and limited resources. Collaborations among decision makers have been identified as critical drivers to improve the efficiency of those decision-making systems, from clinical development phases through to reimbursement of innovative interventions, and, ultimately to accelerate access (Ollendorf et al., 2024). However, the question remains on whether different types of collaborations deliver on their many promises.

A framework to navigate current initiatives
First, we distinguish between horizontal and vertical collaborations. Horizontal collaborations occur among agencies with similar remits and responsibilities (e.g. in the regulatory or HTA space) operating in different countries or jurisdictions.
Regulatory systems, irrespective of maturity and resources, can be more effective if they are willing to leverage collaborative approaches. When CIRS conducted a survey of 32 agencies from Africa, Latin America, and the Middle East (2022), results showed several ways of executing a collaborative approach; 56% were using a collaborative review model and 72% had work-sharing arrangements. There were also regional and centralised reliance models in place (e.g., the Caribbean Public Health Agency (CARPHA)). Figure 1 presents the spectrum of collaborations taking place in the regulatory space.
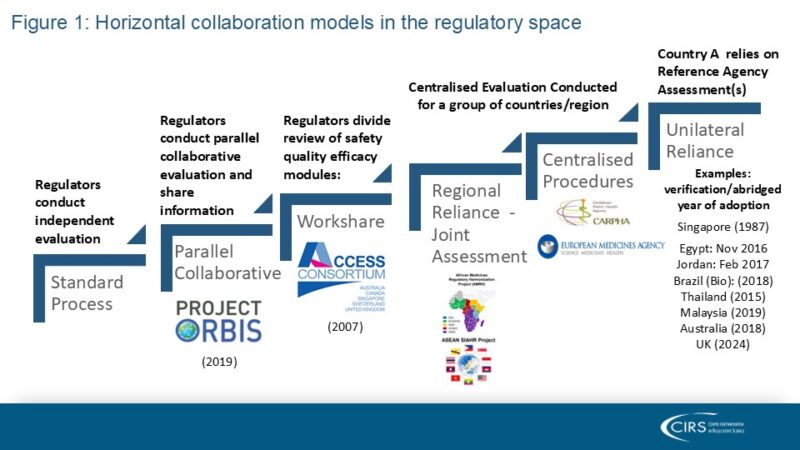
Source: CIRS 2024 Workshop on New ways of working across regulatory and HTA agencies
In the HTA space, collaboration has only started to gain momentum in recent years. While collaboration can encompass various aspects (HTAi Policy Forum, 2024), from professional platforms such as the HTAi Policy Forum to public-private partnerships, this article focuses specifically on collaboration from the perspective of HTA decision making. These collaborations can be part of the same geographical area (such as in the case of the Joint Nordic HTA-Bodies collaboration) or involve sharing assessment approaches (for example, the so-called Commonwealth collaboration between agencies in Australia, Canada, New Zealand and the UK, which operate within the quality-adjusted life year (QALY) paradigm).
In contrast to the matured collaboration models among regulatory agencies, most collaborations of this kind between HTA agencies are voluntary and occupy a different place in a wide spectrum: from sharing experiences and best practices to developing approaches for common challenges (such as surrogate endpoints and modelling treatment pathways), agreeing a standard framework for producing and reporting HTA evidence (e.g. EUnetHTA Core Model), and work sharing in the form of joint assessments of new interventions (such as the upcoming HTA Regulation and the well-established BeNeLuxA initiative). Other possible remits that can be covered in collaborations are common decision making (i.e. making binding recommendations on the use or funding of new interventions in the respective jurisdictions) and sharing work around horizon scanning. Figure 2 presents the types of horizontal collaboration models in the HTA space.
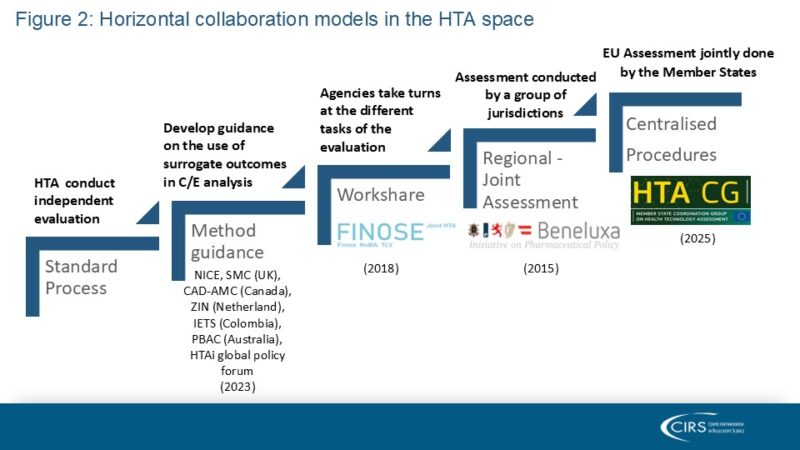
Source: CIRS 2024 Workshop on New ways of working across regulatory and HTA agencies
Vertical collaborations are between agencies with different remits, usually part of different stages of the access pathway. These collaborations can occur upstream during development in the form of joint or parallel scientific advice. In Europe, the EMA-HTA advice was piloted in 2010 and has evolved through EUnetHTA Joint Actions. With the EU HTA Regulation in place, formal Joint Scientific Consultation (JSC) will commence in 2025.
In the downstream decision-making process, regulatory and HTA collaborations have been observed to align timelines and enhance communications. For example, Canada’s aligned pathway between Health Canada, CAD-AMC, and INESSS. In addition, the UK’s Innovative Licensing and Access Pathway (ILAP) offers an integrated approach from upstream development to downstream access, which aims to accelerate patient access to innovative medicines through a collaborative approach involving multiple regulatory and HTA bodies. ILAP provides pharmaceutical companies with access to various tools and services to support efficient evidence development processes.
Final reflections – Collaborations in regulatory and HTA are progressing, but to deliver they need to be better linked via vertical initiatives.
Regulatory agencies worldwide have piloted or implemented several collaborative review initiatives over the past decade, including work sharing, collaborative review, joint assessment, and unilateral reliance. Since no single model fits all situations, a toolkit of several regulatory approaches is needed; these tools are enabled and facilitated by the harmonisation of technical requirements and the adoption of international standards and guidelines.
Meanwhile, a collaborative culture has been steadily developing and expanding within the HTA community. HTA collaborations span a wide range of initiatives, each impacting different aspects of the HTA process, from method development and implementation to joint assessment of specific health technologies. However, the maturity and depth of collaboration among HTA agencies are still at an early stage compared to regulatory collaborations. Collaboration is not achieved overnight; it is a gradual process that depends on trust being built between agencies over time, paving the way for work-sharing and reliance opportunities.
Without vertical collaborations the impact of horizontal collaborations in regulatory can be diluted, e.g. timelines reduced for the licensing process do not necessarily lead to shorter HTA processes if stakeholders do not engage early. For example, CIRS research showed that the rollout times for new medicines approved by Project Orbis were shorter compared to non-Orbis approvals based on shorter submission gap and approval time (CIRS Briefing 88, 2023), thereby demonstrating that global regulatory collaboration can enable faster approval of new therapies for patients with cancer. However, the expedited timelines from initiatives like Project Orbis have highlighted a longer submission gap to HTA agencies following regulatory approval. This is particularly evident with products granted Orbis Type A approvals in Australia and Canada, where the regulatory review is concurrent with FDA with simultaneous submission (less than 30 days from FDA submission) (CIRS briefing 96, 2024). Ongoing discussions among stakeholders, along with coordinated preparations for upcoming pipelines, will be essential to ensure efficient regulatory and HTA decision making.
In terms of vertical collaboration, the benefits of an aligned process through parallel submissions to regulatory and HTA agencies have been evident. Research by CIRS (CIRS briefing 96, 2024) demonstrated that parallel submissions result in an overall shorter timeline compared to sequential submissions. However, no significant influence was observed on HTA decisions, which is logical, given that HTA criteria are not affected by submission timing. Therefore, it is encouraged to better utilise parallel submissions whenever possible to facilitate aligned timing and faster decisions.
Finally, identifying the right remit of these collaborative initiatives is key. There is, for example, a strong case for joining efforts, as part of such collaborations, towards method development, alignment, and implementation to address common challenges, often associated with innovative and complex technologies. This can facilitate and accelerate national processes required for HTA reforms (Radu et al., 2024), also providing a clear signal for developers to inform innovation and evidence generation plans.
The most valuable collaborative initiatives should be identified based on their impact on key measures, such as: contribution to methodological advancements, successful implementation of new methods, improvement in the timeliness of HTA processes, and ultimate impact on patient access to high-value technologies. This will ensure that collaborative initiatives with the most significant impact on healthcare systems and patient outcomes are prioritised.
