The Centre for Innovation in Regulatory Science (CIRS) held a multi-stakeholder workshop that examined current regulatory and reimbursement frameworks for rare disease products (orphan drugs), reviewing how these can be improved to better support the evidence needs of different stakeholders. While incentives have contributed to increased licensing of rare disease products, they are not balanced across rare diseases so refinement may be needed.
Here we summarise workshop outputs from presentations and discussions on incentives for rare disease products, a key focus topic for this workshop.

Background
The development of treatments for rare diseases has been encouraged through regulatory incentives, introduced via legislation, including the US Orphan Drug Act (1983) and EU Regulation EC 141/2000. These provide the opportunity for new treatments in development to receive an orphan drug designation. Incentives can include fee relief, additional scientific advice support and in some cases, a period of exclusive marketing upon marketing authorisation approval from the regulator. However, not all countries have a formal orphan drug designation pathway in place, for example, Canada.
Small patient populations in rare diseases make conducting randomised controlled trials difficult, leading to reliance on alternative evidence sources like real-world evidence for regulatory approval and health technology assessment (HTA) submissions. While regulatory incentives have helped to boost research and development (R&D) for rare disease treatments, the uncertainty in the evidence base poses challenges for HTA agencies and payers.
The European Commission (EC) is currently reforming the legislative framework for the pharmaceutical industry, including regulatory incentives for orphan drugs. Therefore, CIRS felt it well-timed to bring drug stakeholders together in a workshop to evaluate review and reimbursement frameworks for rare disease treatments.
Increased licensing of rare disease treatments
As part of its annual benchmarking study of regulatory agencies, CIRS tracks the approval of drugs with orphan designation in Europe, US, Japan, Switzerland and Australia; some of this data was presented in detail to attendees during the workshop. It showed an increase in approvals of orphan drugs from 2013 to 2022 in these jurisdictions (Figure 1), suggesting that incentives have encouraged R&D in rare diseases.
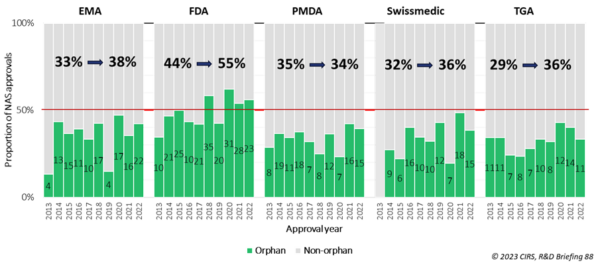
Figure 1: Proportion of new active substance approvals by orphan designation for five major regulatory authorities in 2013-2017 vs 2018-2022. EMA: European Medicines Agency; FDA: US Food and Drug Administration; PMDA: Japanese Pharmaceutical and Medical Devices Agency; TGA: Australian Therapeutic Goods Administration.
Incentives are not balanced across rare diseases
Stakeholders from industry, regulators, HTA agencies, academia and patient organisations shared their views on incentives for rare disease products during the workshop. There is concern that current incentives are not well balanced; while there are orphan drug blockbusters, many rare diseases are neglected in development considerations. There is also the issue of ‘salami slicing’, where diseases are divided into rare subgroups, so incentives can be received for each new indication for the same drug. The EC has proposed changes to incentives for rare disease treatments, leading to concern in the industry that this could lead to a reduction in the development of future treatments. This suggests caution is needed in the pursuit of reforms to orphan drug incentives in Europe.
It was also highlighted that regulatory incentives are not the only incentives in play, with competition in the market influencing pricing and spending on orphan drugs. Perceived high prices and budget impact, with small patient numbers for individual rare diseases adding up as more rare disease treatments come to market, are a concern to some in the HTA and payer community.
Evolving incentives
At each CIRS workshop participants take part in interactive breakout sessions, where multiple stakeholders work together to develop a set of recommendations on a specific aspect of the workshop topic. In this specific workshop, one of the breakouts was tasked with discussing incentives for rare disease products, resulting in the following recommendations:

A summary of all recommendations and key discussion points from the workshop is available in the workshop synopsis.
A full account of the workshop proceedings can be found in the workshop report.
