Timely availability of new medicines to patients is crucial for public health. This process involves several phases: the time taken for regulatory approval, the time to health technology assessment (HTA) submission by the health technology developer, the interval between HTA submission and recommendation, and the subsequent time needed for price negotiations.
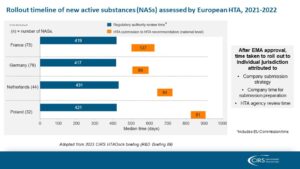
CIRS’s annual HTADock study compared the time from regulatory submission to the first recommendation across four European jurisdictions, shedding light on the duration of each step (see slide below). While the HTA decision time was similar among the four agencies (ranging from 81 to 127 days), the submission strategy for each jurisdiction led to variations in the overall time to HTA decision.
The Regulation (EU) 2021/2282 on HTA (#HTAR) aims to enhance the availability of innovative technologies to EU patients. It represents a significant step toward harmonising clinical assessments in HTA decision making across EU Member States. As the first implementation act takes shape, a definitive procedure and timeline for Joint Clinical Assessment (JCA) submission and production at the European level is becoming apparent. It is important to develop measures to ensure that JCA production facilitates timely rollout of medicines across European jurisdictions.
The next CIRS HTADock study (to be published by June 2024) will provide an update on the current EU regulatory and HTA decision-making timelines, serving as a baseline to reflect future changes as the HTAR comes into force.
This topic will also be further explored at the CIRS multi-stakeholder workshop on 14th June, which aims to discuss the efficient and effective implementation of JCA in national decision making. More information on this workshop can be found in the CIRS Research Agenda.
