HTA Industry Metrics Programme
The landscape of HTA is rapidly evolving globally, driven by improvements in processes and methodologies, as well as policy changes such as the EU HTA Regulation. Over the years, the role of HTA has shifted from a post-approval assessment for reimbursement to an integral part of the entire lifecycle of new medicines.
CIRS has been collaborating with companies through its HTA Industry Metrics Programme to examine the impact of HTA not only on jurisdictional rollout but also during development. Insights gathered from our members help ensure that HTA considerations are embedded into the value proposition throughout a medicine’s lifecycle. A key area of interest for both companies and agencies is the provision of early HTA advice.
In 2025, the programme will focus on analysing the current landscape of early HTA advice. This study will collect data from companies, using consolidated and anonymised information to provide insights into the national dynamics of early HTA advice and establish a baseline to track the evolution of Joint Scientific Consultation (JSC) under the EU HTA Regulation. The study’s findings aim to generate actionable insights that can support decision-making processes related to early HTA.
Key research questions include:
- How many early advice applications were accepted by JSC versus national agencies?
- What types of products undergo early HTA advice?
- Why do some products opt not to seek early advice?
- Which agencies provide early HTA advice?
- Which agencies are most commonly approached for EU Joint Scientific Consultation?
- What topics are typically covered in early HTA advice meetings?
- What are the key feedback points regarding process and procedures?
Additionally, feedback on the early advice process and procedures will be collected and analysed to inform future improvements.
Annual deliverables of the Programme include:
- Executive summary and company-specific report
- Country-specific summaries
- Results of a focused study on a topic of interest to participant companies
- Industry Discussion Meeting to review trends and discuss potential for new analyses
- Periodic updates on the Programme and CIRS advocacy activities
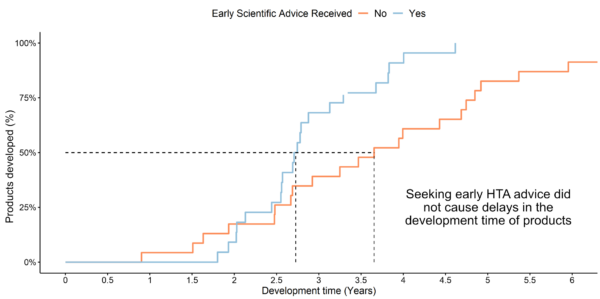
Example analysis: Comparison of development time with and without early HTA advice
(HTA recommendations 2018-2023)
Time from pivotal dose to first regulatory authority submission
