Growth & emerging markets metrics (GEMM) programme
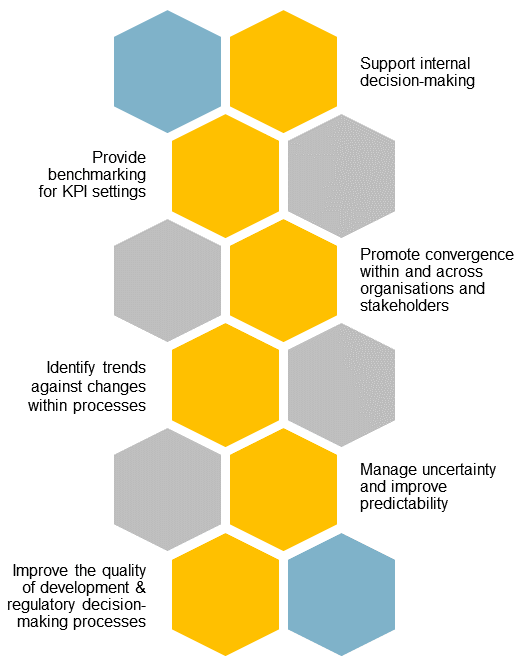
The globalisation of pharmaceutical markets has increased the demand for high-quality information on the development and registration of new medicines in growth and emerging markets. CIRS’ GEMM Programme helps companies navigate these fast-evolving markets by providing comparative regulatory data and insights.
The Programme collects company data annually on:
- Product characteristics
- Regulatory processes and market dynamics
- Submission and approval timelines
- Factors influencing patient access to medicines.
This data is anonymised, aggregated and analysed, providing an industry-wide view of the regulatory landscape across 20 key jurisdictions in Asia, Latin America, the Middle East and Africa. Participating companies can benchmark their performance against industry trends.
Annual Deliverables
- Core Report – A comprehensive 150+ page report with industry-wide and company-specific analyses, aimed at Global Regulatory Leads, Strategy, and Intelligence
- Executive Summary Report – A concise overview of key outcomes, aimed at Management and Policy/Advocacy
- Country Summary Report – Country-specific regulatory snapshots with high-level insights, aimed at Regional Affiliates
- Online Analysis Tool – An interactive, secure platform for benchmarking and analysing programme data, updated annually based on participant feedback
- Industry Discussion Meeting – A forum for discussing trends, regulatory developments, and new analyses based on the most recent data collection
- Company-Specific Analyses* – Bespoke analyses tailored to participants’ reporting needs
- Insight Seminars* – Custom sessions providing updates on CIRS’ activities and discussions on topics of mutual interest related to the Programme
(*Upon request and subject to availability.)