How do HTA timelines and recommendations compare across Canada’s HTA agencies?
At ISPOR Europe 2025, we presented a poster analysing HTA timelines and outcomes for Canada’s Drug Agency (CDA‑AMC) and Institut National d’Excellence en Santé et en Services Sociaux (INESSS) between 2020 and 2023, focusing on 97 common compounds reviewed by both agencies.
Canada’s dual‑agency HTA system provides a useful basis for comparing timelines, submission patterns and recommendation outcomes across jurisdictions. Our analysis examines how CDA‑AMC and INESSS compare on these key elements.
Key insights from our analysis:
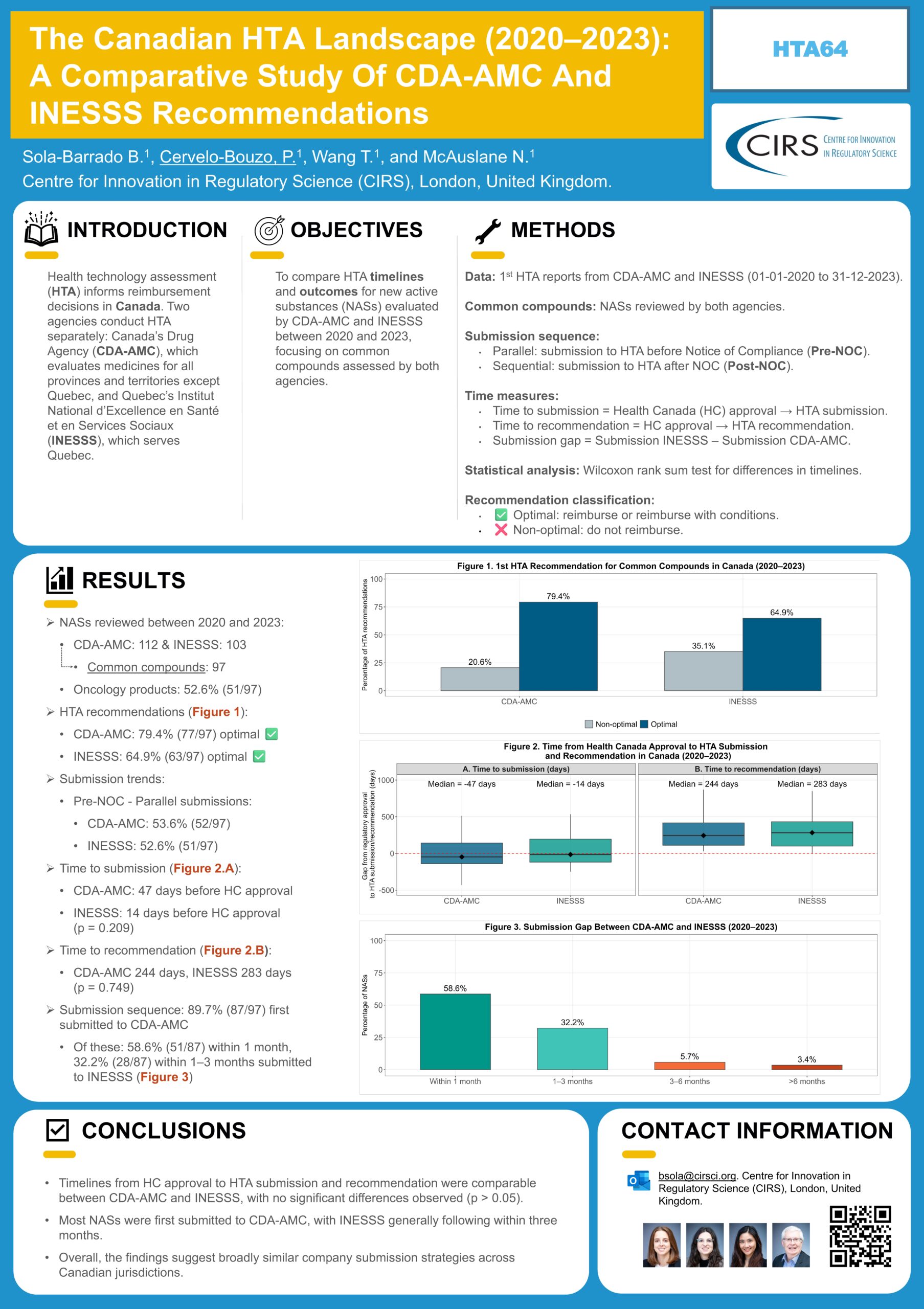
- 97 new active substances (NASs) were submitted to both CDA‑AMC and INESSS; 52.6% of which were oncology products.
- Positive recommendations (with and without restrictions) were more frequent at CDA‑AMC (79.4%) than INESSS (64.9%).
- Timelines from Health Canada approval to HTA submission and recommendation were comparable between CDA‑AMC and INESSS.
- Most NASs were submitted first to CDA‑AMC, with INESSS typically following within 3 months.
- These findings indicate broadly similar company submission strategies across Canadian jurisdictions.
This poster was presented by Penelope Cervelo at ISPOR Europe 2025, 9-12th November 2025, Glasgow, UK.
Conference abstract
Objectives: Health technology assessment (HTA) informs reimbursement decisions in Canada. Two agencies conduct HTA separately: Canada’s Drug Agency (CDA-AMC), which evaluates medicines for all provinces and territories except Quebec, and Quebec’s Institut National d’Excellence en Santé et en Services Sociaux (INESSS), which serves Quebec. To further understand both processes, we compared HTA timelines and outcomes for new active substances (NASs) evaluated by CDA-AMC and INESSS between 2020 and 2023.
Methods: Reports from CDA-AMC and INESSS (01-01-2020 to 31-12-2023) were reviewed. NASs appraised by HTA in both jurisdictions were referred to as common compounds. Time to submission was measured from Health Canada (HC) approval to HTA submission, and time to recommendation was HC approval to HTA recommendation. Differences in time parameters were assessed using the Wilcoxon rank sum test. Recommendations were classified as “optimal” (positive or positive with conditions) or “non-optimal” (negative).
Results: CDA-AMC assessed 108 NASs, and INESSS assessed 103. Of these, 93 NASs received HTA recommendations from both agencies. Among the common NASs, 54% (50/93) were oncology products (ATC code L). Parallel submissions occurred for 52.7% (49/93) and median time to submission was 47 days before HC approval for CDA-AMC and 14 days before for INESSS (p=0.207). The median time to recommendation was 252 days (CDA-AMC) and 286 days (INESSS; p=0.767). Most common compounds (90.3%, 84/93) were first submitted to CDA-AMC. Among these, 58.3% (49/84) were submitted to INESSS within one month, and 32.1% (27/84) within one to three months. Optimal recommendations were issued for 78.5% of common compounds by CDA-AMC versus 65.6% by INESSS.
Conclusions: Timelines from HC approval to HTA recommendation were comparable across agencies. Most submissions were first made to CDA-AMC, with INESSS following within three months. CDA-AMC issued a higher proportion of optimal recommendations. These findings highlight differences in companies’ submission strategies and HTA outcomes across jurisdictions.