To address the complex challenges in the global regulatory environment and the growing demand for patient access to new medicines, regulatory agencies in Asia are actively engaging in regulatory-strengthening and capacity-building initiatives including the use of priority pathways, reliance in the prior reviews of trusted authorities and work sharing to facilitate better utilisation of resources.
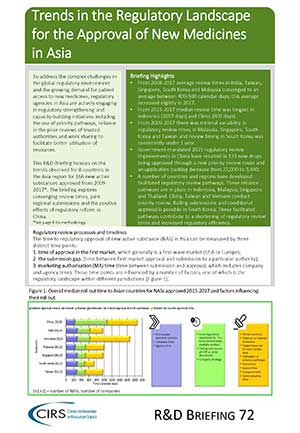
This R&D Briefing focuses on the trends observed for 8 countries in the Asia region for 166 new active substances approved from 2009-2017. The briefing explores converging review times, joint regional submissions and the positive effects of regulatory reform in China.