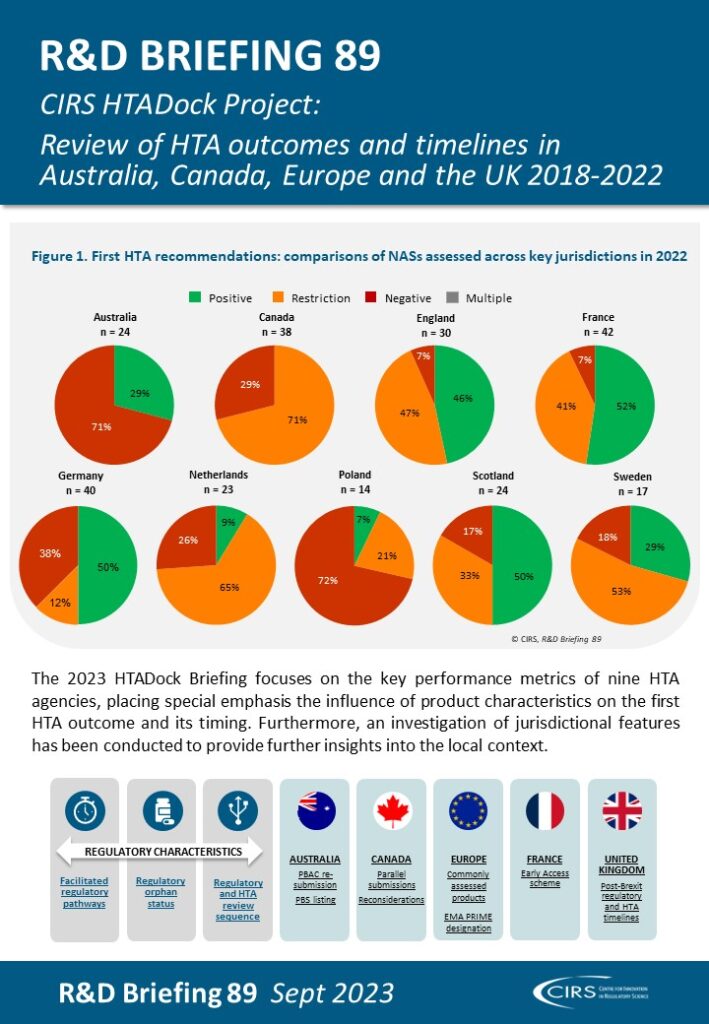
This R&D Briefing presents data from HTADock, an ongoing metrics study that collects publicly available data on new active substances (NASs) appraised by key international HTA agencies, each with unique perspectives and methodologies. The project explores the synchronisation between the regulatory and HTA landscapes, aiming to increase the transparency of the outcomes and timelines of HTA assessments. It also seeks to facilitate the enhancement of performance within HTA agencies.
This year a new jurisdiction was added to the HTADock project: the Netherlands. This brings the total number of HTA agencies included in HTADock to nine:
- Australian Pharmaceutical Benefits Advisory Committee (PBAC)
- Canadian Agency for Drugs and Technologies in Health (CADTH)
- English National Institute for Health and Care Excellence (NICE)
- French Haute Autorité de Santé (HAS)
- German Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG)
- Polish Agencja Oceny Technologii Medycznych i Taryfikacji (AOTMiT)
- National Health Care Institute in Netherlands (ZIN)
- Scottish Medicines Consortium (SMC)
- Swedish Tandvårds & läkemedelsförmånsverket (TLV).
In addition, analyses were undertaken on the potential impact of regulatory work sharing and reliance procedures on HTA, such as Project Orbis and Access in Australia, Canada and the UK, and the European Commission Decision Reliance Procedure (ECDRP) in the UK. Furthermore, the R&D Briefing explores new HTA strategies being implemented in different jurisdictions, such as early access in France.
If you have any questions or comments on this Briefing, please don’t hesitate to get in touch with Tina Wang: twang@cirsci.org